A report which analyses significant developments in the biosimilar space and the impacts on patient access, affordability and quality of care has been released in early 2023. The report is a product of the Patient Access and Affordability Project, a programme of Patients Rising, a non-profit organization.
The report is based on a roundtable discussion with patients, providers, US Food and Drug Administration (FDA)experts, pharmacists and health economists, and addresses all angles of the biosimilar market. It also addresses common patient questions and concerns about the safety and effectiveness of biosimilars, patient access and price controls from the Inflation Reduction Act, and the reasons insurers frequently do not allow patients to participate in cost savings generated by biosimilars.
The report covers significant upcoming trends and milestones of biosimilars for chronic disease patients that are expected to happen in 2023. These events involve the introduction of a large range of biosimilars of the anti-inflammatory drug Humira (adalimumab), which is currently the world’s top-selling drug. There could be as many as 10+ biosimilar competitors on the market by the end of 2023, based on current FDA approvals and pending approvals [1, 2].
Some biosimilar manufacturers obtain an ‘interchangeable’ designation from FDA. These biosimilars can be substituted for the reference product at the pharmacy without prior approval from the doctor, based on state law. Patients should consult with their doctor and pharmacist to understand the rules in their state and ask questions before switching to a biosimilar.
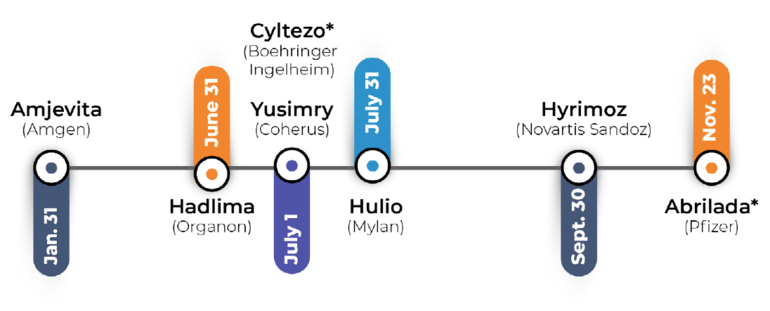
Boehringer-Ingelheim’s Cyltezo will be the first Humira biosimilar to be launched in July 2023 with an ‘interchangeable’ designation from FDA, Pfizer’s Abrilda will be the second set for release in December 2023, see Figure 1.
Figure 1: Humira biosimilars launches in 2023§
§as of 15 January 2023
Patients on Humira or other originator biologicals should consult with their doctor before switching to a biosimilar, whether due to insurance coverage or cost. Biosimilars are rigorously tested by FDA and must demonstrate the same safety and efficacy as the originator product with no meaningful differences. Doctors can prescribe any medication they believe is appropriate for their patients, regardless of any interchangeable designation from FDA.
Biosimilars have the potential to bring significant savings to the health system, but insurance plans may not allow patients to fully participate in these savings. The next phase of the biologicals market, with increased biosimilar competition, will determine how insurance plans and health systems handle this issue. When a new biosimilar becomes available, there may be significant differences among insurance coverage and preference tiers. It can be difficult for patients to determine if switching to a biosimilar offers both treatment and financial benefits. Patients currently on biologicals should feel confident in FDA-approved biosimilars, but should consult with their doctor to understand the impact on their treatment. Physicians should also help ease concerns about differences between originator biologicals and biosimilars. Patients should pay close attention and frequently consult with their doctors to better understand how switching to a biosimilar will impact their treatment.
Related articles
US PBMs add multiple Humira biosimilars to formularies
Market opportunities for biosimilars
References
1. GaBI Online - Generics and Biosimilars Initiative. New adalimumab biosimilars prepare to launch in Canada, US and Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 May 19]. Available from:
www.gabionline.net/biosimilars/news/new-adalimumab-biosimilars-prepare-to-launch-in-canada-us-and-europe
2. GaBI Online - Generics and Biosimilars Initiative. Humira adalimumab biosimilars pipeline [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 May 19]. Available from:
www.gabionline.net/reports/humira-adalimumab-biosimilars-pipeline
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment