The United Kingdom’s (UK) Medicines and Healthcare products Regulatory Agency (MHRA) and South Korea’s Ministry of Food and Drug Safety have approved biosimilar versions of Genentech’s ophthalmology drug, Lucentis (ranibizumab).
Ranibizumab is a monoclonal antibody fragment created from the same parent mouse antibody as bevacizumab. Ranibizumab inhibits angiogenesis (the formation of new blood vessels) by inhibiting vascular endothelial growth factor alpha (VEGF-A), a mechanism similar to bevacizumab [1, 2].
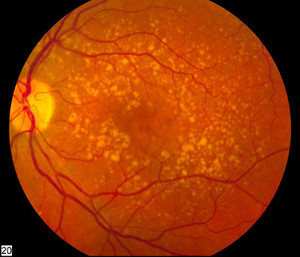
Ranibizumab can be used to treat macular degeneration by inhibiting VEGF, which is responsible for the excessive formation of blood vessels in the retina leading to progressive loss of vision. The monoclonal antibody drug is indicated for the treatment of wet age-related macular degeneration (AMD), macular oedema, degenerative myopia and diabetes complications; all conditions of the eye causing vision loss [1].
The patents on Lucentis expired in the US in June 2020 and will expire in Europe in 2022 [3].
The ranibizumab biosimilar approved in the UK is Israeli generics giant Teva Pharmaceuticals’ (Teva) Ongavia, produced in partnership with Bioeq and Formycon. This has been approved for the treatment of neovascular (wet) AMD. age-related macular degeneration (AMD). Ongavia is also licensed for the treatment of visual impairment due to diabetic macular oedema (DME); the treatment of proliferative diabetic retinopathy (PDR); the treatment of visual impairment due to macular oedema secondary to retinal vein occlusion (branch RVO or central RVO); and the treatment of visual impairment due to choroidal neovascularization (CNV).
Teva entered into a strategic partnership on 28 June 2021 with Bioeq for the exclusive commercialization of Bioeq’s ranibizumab biosimilar (FYB201) in Europe, Canada, Israel and New Zealand [4].
Formycon and Bioeq announced a European marketing authorization application for ranibizumab biosimilar, FYB201 [5]; and Bioeq submitted an application for ranibizumab biosimilar CHS-201 (also known as FYB201) to the US FDA [6].
In Korea, Samsung Bioepis received marketing approval for Amelivu (ranibizumab) that also references Lucentis. However, market release of the treatment will be delayed due to a patent-related agreement between Samsung Bioepis and Genentech.
The Samsung Bioepis’ biosimilar ranibizumab has already been approved in the US [7], Europe [8] and Canada [9], under the brand name, Byooviz.
Related articles
Fresh partnerships announced for ranibizumab and trastuzumab biosimilars
Biosimilars approved in South Korea
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View last week’s headline article: Nomenclature of biologicals and biosimilars in Peru Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de la semana pasada: Nomenclature of biologicals and biosimilars in Peru !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of ranibizumab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/biosimilars/general/Biosimilars-of-ranibizumab
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of bevacizumab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-of-bevacizumab
3. Derbyshire M. Patent expiry dates for biologicals: 2018 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(1):24-31. doi:10.5639/gabij.2019.0801.003
4. GaBI Online - Generics and Biosimilars Initiative. Teva signs deal with Bioeq for ranibizumab biosimilar FYB201 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/pharma-news/teva-signs-deal-with-bioeq-for-ranibizumab-biosimilar-fyb201
5. GaBI Online - Generics and Biosimilars Initiative. Formycon/Bioeq submit European marketing authorization for ranibizumab biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/biosimilars/news/formycon-bioeq-submit-european-marketing-authorization-for-ranibizumab-biosimilar
6. GaBI Online - Generics and Biosimilars Initiative. Bioeq submits application for ranibizumab biosimilar to FDA [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/biosimilars/news/bioeq-submits-application-for-ranibizumab-biosimilar-to-fda
7. GaBI Online - Generics and Biosimilars Initiative. EC and FDA approval for first ranibizumab biosimilar Byooviz [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/biosimilars/news/ec-and-fda-approval-for-first-ranibizumab-biosimilar-byooviz
8. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/biosimilars/general/biosimilars-approved-in-europe
9. GaBI Online - Generics and Biosimilars Initiative. Canada approves ranibizumab biosimilar Byooviz [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 May 27]. Available from: www.gabionline.net/biosimilars/news/canada-approves-ranibizumab-biosimilar-byooviz
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2022 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment