In early 2023, the United States Food and Drug Administration (FDA) published their Biosimilar User Fee Act (BsUFA) III research roadmap [1] as part of their regulatory research pilot programme [2].
BsUFA III spans fiscal years FY 2023‒2027 and builds on BsUFA I and II. Respectively, I and II enabled the development of the initial biosimilar review programme infrastructure and focused on creating ‘effective scientific coordination and review consistency through review, procedural, and meeting performance enhancements’.
BsUFA III includes additional steps to ensure efficient governance and operations across the biosimilar product review programmes. As part of BsUFA III, FDA committed to ‘pilot a regulatory science research programme to further enhance regulatory decision-making and facilitate science-based recommendations in areas foundational to biosimilar development’. The recently published research roadmap comes under the remit of this commitment.
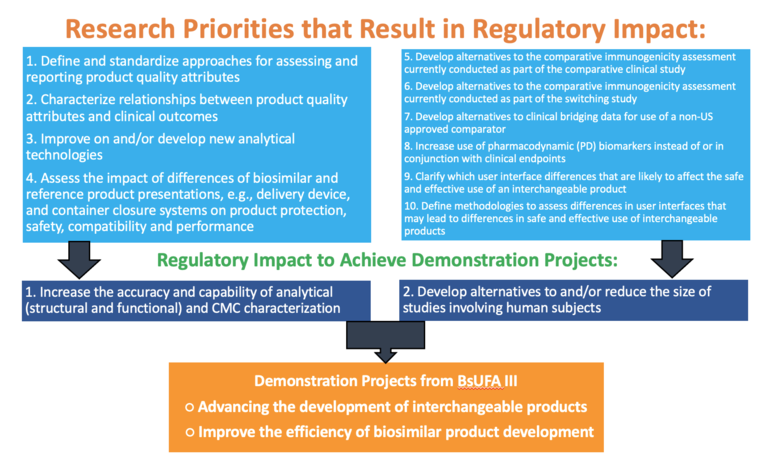
The research roadmap features two demonstration projects:
-
Advancing the development of interchangeable products
o Focuses on generating information and methodologies to meet the safety standards for establishing interchangeability. With emphasis on assessing differences in product presentations and container closure systems and predicting immunogenicity.
-
Improving the efficiency of biosimilar product development
o Works towards streamlining the biosimilar development process and will investigate methodology development to predict immunogenicity and conduct analytical and pharmacological assessments.
The roadmap has 10 research priorities that will result in regulatory impact and enable achievement of the two demonstration projects. These are shown in Figure 1. Methods to consider for research conducted as part of the pilot programme are: analytical methods; biological assays; efficient clinical design, e.g. statistical methods; in silico/in-vitro modelling; model-informed drug development (MIDD) applications; pharmacological studies; and real-world date/evidence (RWE/RWD)
Figure 1: FDA BsUFA III research roadmap on regulatory impact
Overall, the aim of the pilot programme is to rework the regulatory process to focus on more comparative analytical assessments and less on the clinical pharmacology assessments and comparative clinical studies.
As outlined in their commitment letter, FDA aims to provide interim progress reports and workshops in 2025 and a final summary report in 2027. In addition, a strategy document will be produced to outline the actions FDA will take to facilitate the development of biosimilars and interchangeable products.
Related articles
Biosimilar User Fee Act (III) performance goals letter published
FDA publishes final Q&A on biosimilar development and the BPCI Act
Biosimilar User Fee Act reauthorisation
FDA issues new guidance on biosimilar user fees
|
LATIN AMERICAN FORUM
The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View the latest headline article: Directrices revisadas de la OMS para productos biosimilares seguros y eficaces Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO
Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Ver el último artículo de cabecera: Directrices revisadas de la OMS para productos biosimilares seguros y eficaces !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. U.S. Food and Drug Administration. BsUFA III Regulatory Research Pilot Program: Research Roadmap [homepage on the Internet]. [cited 2023 Mar 3]. Available from: https://www.fda.gov/media/164751/download
2. GaBI Online - Generics and Biosimilars Initiative. FDA funds regulatory science pilot for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Mar 3]. Available from: www.gabionline.net/policies-legislation/fda-funds-regulatory-science-pilot-for-biosimilars
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment