Global spending on medicine continues to grow. As of 2019, approximately US$1.25 trillion had been spent on medicines, up from just US$887 billion in 2010. That number is expected to increase to US$1.59 billion by 2024, with biologicals making up an increasing part of this total due to their use to treat previously intractable diseases. In fact, spending on biologicals has doubled since 2007 and growth has outstripped that of total sales of pharmaceuticals by a significant margin. The global biologicals market in 2020 was worth approximately US$239.2 billion, and in 2020 five of the top 10 best-selling drugs were biologicals compared with a high of seven in 2018, and only three in 2008 [1].
The increasing cost of medicines, and especially biologicals, is putting increasing strain on healthcare budgets and is therefore pushing the growth in the biosimilars market share. In Europe, the share of the European Union’s biologicals market that is subject to competition from biosimilars increased from 9% back in 2013 to 29% in 2018 [2]. The sales of biosimilars have now reached €8.4 billion, which represents 9% of the total biologicals market in 2020, reflecting their value for money [3].
However, the situation in the US is not quite so rosy. There is currently only 19% of the biologicals market, or US$40 billion, already facing some biosimilar competition and biosimilars currently make up only 2.3% of the US biologicals marketplace. In fact, 90% of global biosimilars sales take place in Europe, in spite of 60% of overall biologicals sales occurring in the US [4].
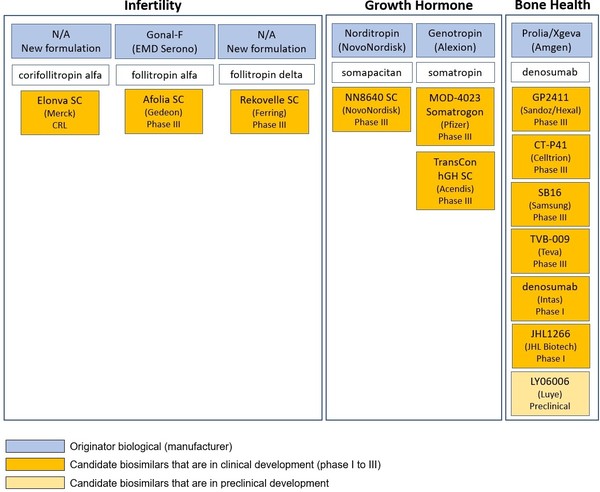
Despite this lack of uptake of biosimilars in the US, the pipeline for biosimilars still looks healthy. In fact, in the areas of growth hormones and infertility, there are 13 biosimilars in the pipeline for the US biosimilar marketplace [5], see Table 1.
Growth hormone is a protein hormone of about 190 amino acids that is synthesized and secreted by cells called somatotrophs in the anterior pituitary. It is a major participant in control of several complex physiologic processes, including growth and metabolism. Biologicals such as Genotropin (somatropin) are used to treat growth failure in children and adults who lack natural growth hormone.
Infertility treatments such as Gonal-F are generally naturally occurring hormones that are used to stimulate a follicle (egg) to develop and mature. Gonal-F is used when a woman desires pregnancy and her ovaries can produce a follicle but hormonal stimulation is not sufficient to make the follicle mature.
Bone health covers treatments for osteoporosis, treatment-induced bone loss and metastases to bone, such as denosumab. Denosumab is a humanized monoclonal antibody that is an inhibitor of the receptor activator of nuclear factor kappa-B ligand (RANKL), which works by preventing the development of osteoclasts which are cells that break down bone.
Table 1: US pipeline for infertility, growth hormone and bone health biosimilars
CRL: contract research laboratory; SC: subcutaneous.
Source: GaBI Online Biosimilars of denosumab, somatropin, somapactin, corfollitropin alfa, follitropin alfa, follitropin delta.
Related articles
US biosimilars pipeline for immunosuppressants, insulin and ophthalmology – 2021
US biosimilars pipeline for supportive care, oncology and TNF inhibitors – 2021
Approval and launch dates for US biosimilars – 2021
| LATIN AMERICAN FORUM – Coming soon! To further enhance the objectives of GaBI in sharing information and knowledge that ensure policies supportive of safe biosimilars use, we are pleased to announce that we will be launching a new section on GaBI Online and GaBI Journal, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America. Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM – Próximamente! Para fomentar los objetivos de GaBI sobre la difusión de información y conocimiento sobre las políticas de apoyo que garantizan el uso seguro de medicamentos biosimilares, nos complace anunciar el lanzamiento de una nueva sección en GaBI Online y GaBI Journal, el ‘Latin American Forum’ (en español), que presentará las últimas noticias y actualizaciones en investigación y desarrollo sobre medicamentos genéricos y biosimilares en Latinoamérica. Regístrese para recibir el boletín informativo GaBI Latin American Forum. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. Derbyshire M, Shina S. Patent expiry dates for best-selling biologicals: 2018 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(1):24-31. doi:10.5639/gabij.2019.0801.003
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars market and opportunities in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 May 31]. Available from: www.gabionline.net/Reports/Biosimilars-market-and-opportunities-in-Europe
3. European Commission. Troein P, Newton M, Scott K. The impact of biosimilar competition in Europe. December 2020 [homepage on the Internet]. [cited 2021 May 28]. Available from: https://ec.europa.eu/health/sites/default/files/human-use/docs/biosimilar_competition_en.pdf
4. GaBI Online - Generics and Biosimilars Initiative. The sluggish US biosimilars market [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 May 28]. Available from: www.gabionline.net/Reports/The-sluggish-US-biosimilars-market
5. McGowan S, Jesse M, Biehn B. U.S. Biosimilar Report. AmerisourceBergen, April 2021.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Source: AmerisourceBergen








 0
0











Post your comment