Last update: 4 December 2020
Ranibizumab is a monoclonal antibody fragment created from the same parent mouse antibody as bevacizumab. Ranibizumab inhibits angiogenesis (the formation of new blood vessels) by inhibiting vascular endothelial growth factor A (VEGF-A), a mechanism similar to bevacizumab [1].
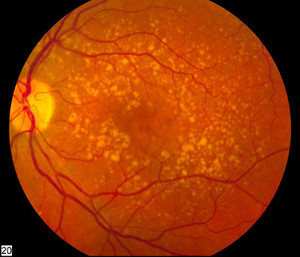
Ranibizumab can be used to treat macular degeneration by inhibiting VEGF, which is responsible for the excessive formation of blood vessels in the retina leading to progressive loss of vision. The monoclonal antibody drug is indicated for the treatment of wet age-related macular degeneration (AMD), macular oedema, degenerative myopia and diabetes complications; all conditions of the eye causing vision loss.
The originator product, blockbuster wet AMD treatment Lucentis (ranibizumab) marketed by Genentech (Roche)/Novartis, was approved by the US Food and Drug Administration (FDA) in June 2006 and by the European Medicines Agency (EMA) in January 2007 [2]. Lucentis had estimated global sales of approximately US$3.6 billion in 2015.
The patents on Lucentis will expire in the US in June 2020 and in Europe in 2022 [1]. Some of the ranibizumab biosimilars and non-originator biologicals* approved or in development are presented in Table 1.
| Table 1: Biosimilars and non-originator biologicals* of ranibizumab approved or in development
|
| Company name, Country
|
Product name
|
Stage of development
|
| BIOCND, South Korea
|
BCD300
|
Phase 1 trial expected to be completed by end 2017. Marketing approval expected by 2020.
|
| Biocure, South Korea
|
-
|
Preclinical [3]
|
| Biocon/Samsung Bioepis, USA/South Korea
|
SB11
|
Collaborating with C-Bridge Capital (AffaMed Therapeutics) for copy biologicals in China [4]. Phase III trial in AMD ongoing. Submitted to EMA and FDA for approval in October and November 2020, respectively [5, 6].
|
| Chong Kun Dang Pharmaceutical, South Korea
|
CKD 701
|
Preclinical
|
| Coherus, USA
|
CHS-3351
|
Preclinical [2]
|
| Formycon/Bioeq IP (Santo Holding/ Polpharma), Germany
|
FYB201
|
Phase III trial started in October 2015 [7]
|
| Hospira (Pfizer)/Pfenex, USA
|
PF582
|
Entered into an agreement to exclusively develop and commercialize PF582 in February 2015 [8]. Pilot phase I/II study in AMD ongoing as of February 2015.
|
| Intas Biopharmaceuticals, India*
|
Razumab
|
‘Similar biologic’ launched in India in June 2015 [9]
|
| Polus Biopharm, South Korea
|
PDP807
|
Secondary target
|
| Siam Bioscience, Thailand
|
SBS 7001
|
Positive data from in vitro biosimilarity study reported in September 2017
|
| Xbrane Biopharma/Stada Arzneimittel, Sweden/Germany
|
Xlucane
|
Positive data from in vitro biosimilarity study comparing Xlucane versus Lucentis reported in February 2017 [10]. Study design for pivotal phase I/III clinical trial in wet AMD agreed with EMA and FDA [11]
|
AMD: age-related macular degeneration.
*See editor’s comment
|
Editor’s comment
European Medicines Agency regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product but they are not universally accepted by regulatory bodies outside of the European Union (EU). It should be noted that 'copy biologicals’ approved in China and ‘similar biologics’ approved in India might not have been authorized if they had been subjected to the strict regulatory processes required for approval of biosimilars in the EU.
Related articles
Biosimilars of rituximab
Biosimilars of infliximab
Biosimilars of adalimumab
Biosimilars of trastuzumab
Biosimilars of cetuximab
LATIN AMERICAN FORUM
We are pleased to announce, that starting January 2021, the launch of a new section on GaBI Online, the ‘Latin American Forum’ (in Spanish) featuring the latest news and updates on research and developments in generic and biosimilar medicines in Latin America.
Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
|
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of bevacizumab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Dec 6]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-of-bevacizumab
2. Derbyshire M. Patent expiry dates for biologicals: 2018 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2019;8(1):24-31. doi:10.5639/gabij.2019.0801.003
3. GaBI Online - Generics and Biosimilars Initiative. Biocure developing five candidate biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/General/Biocure-developing-five-candidate-biosimilars
4. GaBI Online - Generics and Biosimilars Initiative. Samsung Bioepis makes deals for copy biologicals in China [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Dec 6]. Available from: www.gabionline.net/Pharma-News/Samsung-Bioepis-makes-deals-for-copy-biologicals-in-China
5. GaBI Online - Generics and Biosimilars Initiative. EMA accepts application for ranibizumab biosimilar from Samsung Bioepis [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/News/EMA-accepts-application-for-ranibizumab-biosimilar-from-Samsung-Bioepis
6. GaBI Online - Generics and Biosimilars Initiative. FDA accepts application for ranibizumab biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2020 Dec 4]. Available from: www.gabionline.net/Biosimilars/News/FDA-accepts-application-for-ranibizumab-biosimilar
7. GaBI Online - Generics and Biosimilars Initiative. Formycon starts phase III trial for ranibizumab biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Oct 30]. Available from: www.gabionline.net/Biosimilars/News/Formycon-starts-phase-III-trial-for-ranibizumab-biosimilar
8. GaBI Online - Generics and Biosimilars Initiative. Hospira and Pfenex to collaborate on ranibizumab biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Oct 30]. Available from: www.gabionline.net/Biosimilars/News/Hospira-and-Pfenex-to-collaborate-on-ranibizumab-biosimilar
9. GaBI Online - Generics and Biosimilars Initiative. Ranibizumab similar biologic launched in India [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Oct 30]. Available from: www.gabionline.net/Biosimilars/News/Ranibizumab-similar-biologic-launched-in-India
10. GaBI Online - Generics and Biosimilars Initiative. Advances in teriparatide and ranibizumab biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2017 May 12]. Available from: www.gabionline.net/Biosimilars/News/Advances-in-ranibizumab-and-teriparatide-biosimilars
11. GaBI Online - Generics and Biosimilars Initiative. Licensing and co-development deals for ranibizumab and adalimumab biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2019 Dec 6]. Available from: www.gabionline.net/Pharma-News/Co-development-deal-for-ranibizumab-biosimilar-Xlucane
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2015 Pro Pharma Communications International. All Rights Reserved.
Source: ClinicalTrials.gov, EMA, US FDA








 2
2










Post your comment