Biosimilars/Research
|
Posted 17/09/2021
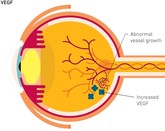
The increasing global burden of chronic diseases, including cancers, diabetes, autoimmune disorders, anaemia of chronic renal failure, rheumatoid arthritis, multiple sclerosis, blood disorders and others, underscore the importance of patients’ access to safe and effective treatments. Interestingly, the introduction of biologicals in the 1980s revolutionized the treatment of these chronic diseases with better prognosis, although high costs and limited patient access remain challenges. These biologicals are known by various names, including biopharmaceutical agents, biologicals, biological therapies, biological agents and biological response modifier therapy or immunotherapy. Biologicals are derived or manufactured from a living biological system. With the majority of originator biologicals losing patent protection and the emergence of biosimilars, the landscape of biologicals is facing many changes.