On 20 May 2024, the US Food and Drug Administration (FDA) approved Biocon Biologic’s Yesafili (aflibercept-jbvf) and Samsung Bioepis and Biogen's Opuviz (aflibercept-yszy) as the first interchangeable biosimilars to Regeneron and Bayer's Eylea (aflibercept). The Samsung Bioepis product has also been launched in South Korea under brand name, Afilivu.
Aflibercept is a vascular endothelial growth factor (VEGF) inhibitor [1], a recombinant fusion protein consisting of human VEGF receptor 1 and VEGF receptor 2 extracellular domains fused to the Fc portion of human IgG1 which acts as a soluble decoy for the natural VEGF receptors that inhibits their activation, thereby reducing pathological angiogenesis.
Both Yesafili and Opuviz are used to treat:
- Neovascular (wet) age-related macular degeneration
- Macular oedema following retinal vein occlusion
- Diabetic macular oedema
- Diabetic retinopathy
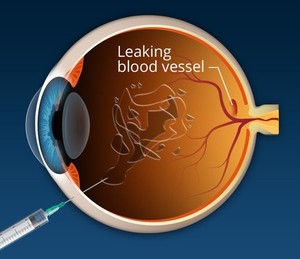
Both Yesafili and Opuviz are administered intravitreally (in the eye) as a 2 mg (0.05 mL of 40 mg/mL) injectable solution to treat patients for the conditions listed above, according to dosing regimens recommended in the labelling. Yesafili was approved in Europe in September 2023, and Biocon is set to commercialize its biosimilars in 31 European countries [2, 3].
In South Korea, Afilivu (aflibercept), an Eylea biosimilar, was launched on 1 May 2024. This is Samsung Bioepis’ second biosimilar for ophthalmic disease, after its Lucentis (ranibizumab) biosimilar, Amelivu, launched in Korea in 2023 [4].
Related articles
Highlights of EMA approvals in 2023 focus on cancer medicines
Ophthalmology biosimilars in Canada: a prescriber’s perspective
Biosimilars approved in South Korea
|
LATIN AMERICAN FORUM
The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View the latest headline article: Actualización de los biosimilares aprobados por Health Canada en 2023 Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO
Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Ver el último artículo de cabecera: Actualización de los biosimilares aprobados por Health Canada en 2023 !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of aflibercept [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Jun 19]. Available from: www.gabionline.net/biosimilars/general/biosimilars-of-aflibercept
2. GaBI Online - Generics and Biosimilars Initiative. EC approval of natalizumab, aflibercept and tocilizumab biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Jun 19]. Available from: www.gabionline.net/biosimilars/news/ec-approval-of-natalizumab-aflibercept-and-tocilizumab-biosimilars
3. GaBI Online - Generics and Biosimilars Initiative. Biocon to commercialize biosimilars in 31 European countries biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Jun 19]. Available from: www.gabionline.net/pharma-news/biocon-to-commercialize-biosimilars-in-31-european-countries
4. GaBI Online - Generics and Biosimilars Initiative. Ranibizumab biosimilar Ximluci and Amelivu to launch in the UK and South Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Jun 19]. Available from: www.gabionline.net/biosimilars/news/ranibizumab-biosimilar-ximluci-and-amelivu-to-launch-in-the-uk-and-south-korea
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2024 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment