In a presentation based on the report by health information technology and clinical research company IQVIA ‘Biosimilars in the US 2020–2024: Competition, savings and sustainability’ biosimilars launched, approved or in development for the US market were discussed [1].
The presentation, which was given by Doug Long of IQVIA at the AAM 2021 Annual Meeting, outlined how the US biologicals market continues to grow faster than non-biologicals on an invoice-basis and that it now comprises 43% of spending in the US. In fact, between 2015 and 2019 the US biologicals market grew by a compound annual growth rate (CAGR) of 14.6% compared to the total medicines CAGR of 6.1% and for small molecules of 1.6%. Despite this, small molecules still accounted for the majority of the US$493 billion spent on medicines in the US in 2019: 57% compared to 43% for biologicals.
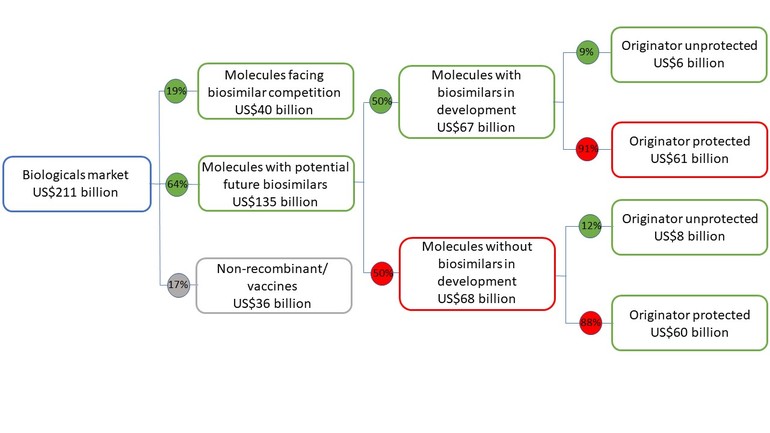
However, this may change in the future as molecules with biosimilars in the US now total US$40 billion of invoice spending, while biosimilar development is targeting a further US$67 billion, see Figure 1.
Figure 1: 2019 US biologicals market, including biosimilar competition, development and market exclusivity
Source: Biosimilars in the US: Competition, savings and sustainability. Report by the IQVIA Institute for Human Data Science.
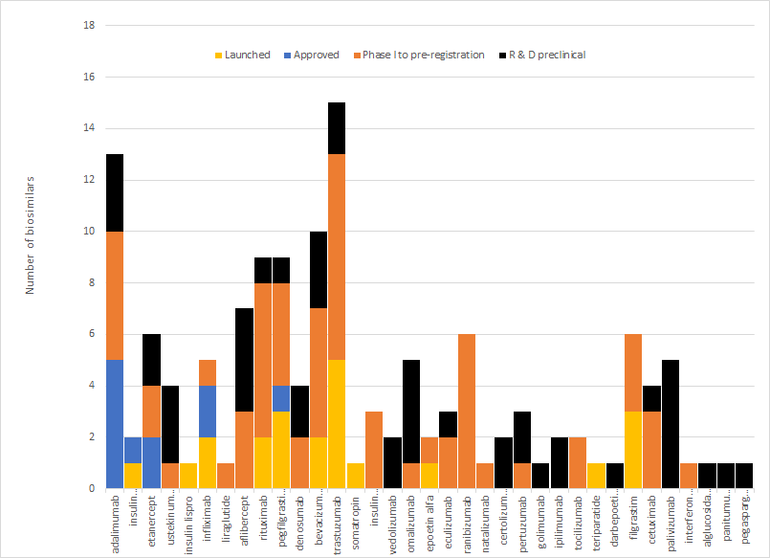
In fact, up to September 2021 there have been 29 approvals across 13 molecules [2], although only 18 have so far been launched in the country [3]. In addition, there are a further 108 biosimilars in development across 22 other molecules. This gives a total for biosimilars launched, approved or in development of 35 different molecules, see Figure 2.
Figure 2: Biosimilars approved, launched or in development by molecule
Source: Biosimilars in the US: Competition, savings and sustainability. Report by the IQVIA Institute for Human Data Science.
Note: Biologicals arranged decreasing order according to US spending in 2019.
Currently, 13% of biosimilars in development are being developed by six large pharma companies. The remaining 87% are being developed by 41 smaller companies with varying degrees of biological or biosimilar development experience. For biosimilars marketed in the US, 14 were developed and launched by seven large pharma companies.
Related articles
Biosimilar launches and uptake expected to increase in the US
US savings from biosimilars could exceed US$100 billion
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View this week’s headline article: Posible colaboración biotecnológica entre India y Colombia Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de esta semana: Posible colaboración biotecnológica entre India y Colombia Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. IQVIA. Biosimilars in the US 2020-2024: Competition, savings and sustainability. Report by the IQVIA Institute for Human Data Science. 29 September 2020.
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Oct 15]. Available from: www.gabionline.net/biosimilars/general/Biosimilars-approved-in-the-US
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilar approvals and launches in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Oct 15]. Available from: www.gabionline.net/biosimilars/general/Biosimilar-approvals-and-launches-in-the-US
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment