First posted: 9 August 2012
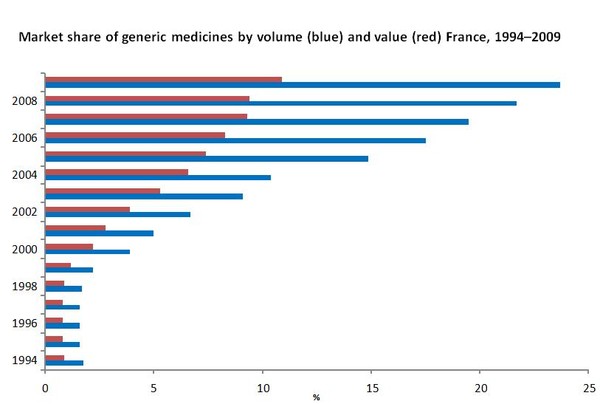
France had an undeveloped generic medicines market during the second half of the 1990s. This changed with the introduction of incentives for physicians and pharmacists in the early 2000s. Market share of generic medicines has grown from 0.9% in 1994 to 10.9% in 2009 in terms of value of consumption and from 1.8% in 1994 to 23.7% in 2009 in terms of volume of consumption [1, 2].
Source: 1994–2004 [1]; 2005–2008 [2]
France has pricing regulation of generic medicines, which means that penetration of generic medicines is less successful than in countries that permit (relatively) free pricing of medicines, e.g. Germany, The Netherlands, UK [3].
Reference-pricing systems appear to have aided the development of national generic medicines markets by imposing a patient co-payment on originator medicines priced above the level of the reference price. However, the primary objective of a reference-pricing system is to contain public medicines expenditure, not to stimulate generic medicines use. However in France, where the introduction of the reference-pricing system was accompanied by price reductions of many originator medicines to the level of the reference price, the contribution of the reference-pricing system to the development of the generic medicines market was limited [1].
Prescribing by international non-proprietary name is voluntary for physicians in France [4] and there are no other major incentives for them to prescribe generic products.
In 2010, the generics substitution rate by pharmacists averaged 80% nationally [5].
Patient co-payments tend to be covered by private insurance, therefore no incentive exists for patients to demand cheaper (generic) medicines [1].
The social health insurance (Délégués d’Assurance Maladie, DAM) organises information activities on a regular basis targeted at patients in order to increase awareness of generic medicines [6].
Highlights of the generics market in France
- Generics substitution in France produced savings of Euros 1 billion for the health insurance system in 2008 [7].
- Market share of generic medicines by volume is 23.7%, accounting for 10.9% in value [2].
- Some policy measures to encourage physicians to prescribe generics have been taken in recent years [1].
- Substitution of generics for originator medicines is financially attractive to pharmacists [1].
- However, law prohibits the automatic substitution of one biological medicine for another without the consent of the treating physician. The reason given is that innovator biotech products and follow-on medicines are not identical [1].
References
1. Simoens S, De Coster S. Sustaining generic medicines markets in Europe. 2006 Apr. [monograph on the Internet]. Brussels, Belgium, European Generic medicines Association (EGA) [cited 2012 Jun 10]. Available from: www.egagenerics.com/doc/simoens-report_2006-04.pdf
2. Agence française de sécurité sanitaire des produits de santé, AFSSAPS (French Medicines Agency). Les ventes de medicaments aux officines et aux hôpitaux en France: chiffres-clés 2009. [Sales of drugs to pharmacists and hospitals in France: key figures 2009]. 6th ed. 2010 Oct. French.
3. Simoens S. Generic medicine pricing in Europe: current issues and future perspective. J Med Econ. 2008;11(1):171-5.
4. Österreichisches Bundesinstitut für Gesundheitswesen (ÖBIG). Surveying, assessing and analysing the pharmaceutical sector in the 25 EU Member States. 2006 Jul.
5. Mutualité Française. Favoriser les génériques dans tous les départments. [Promote generics in all departments]. [monograph on the Internet]. Paris, France, Mutualité Française [cited 2012 Jun 10]. French. Available from: www.mutualite.fr/L-actualite/Kiosque/Revues-de-presse/Favoriser-les-generiques-dans-tous-les-departements/(language)/fre-FR
6. Vogler S, Schmickl B. Rational use of medicines in Europe. Gesundheit Österreich GmbH/Österreichisches Bundesinstitut für Gesundheitswesen (GÖG/ÖBIG). 2010 Feb.
7. Mutualité Française. Le succès des génériques. [The success of generics]. [monograph on the Internet]. Paris, France, Mutualité Française [cited 2012 Jun 10]. French. Available from: www.mutualite.fr/L-actualite/Kiosque/Revues-de-presse/Le-succes-des-generiques/(language)/fre-FR








 0
0






Post your comment