Shanghai Henlius Biotech, Inc (Henlius) reports progress in clinical trials of a bevacizumab copy biological for age-related macular degeneration and colorectal cancer, while Innovent Biologics reports positive findings from a trial of their bevacizumab copy biological in hepatocellular carcinoma.
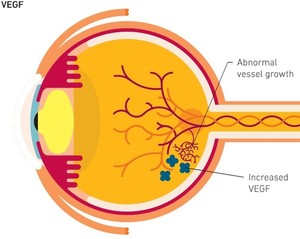
Bevacizumab is a monoclonal antibody targeting vascular endothelial growth factor (VEGF). It can be used to treat certain forms of cancer as well as wet age-related macular degeneration (AMD), which is a leading cause of visual impairment and blindness.
On 19 July 2021, China’s Henlius announced that the first patient had been dosed in a phase I clinical trial for their bevacizumab copy biological, HLX04-O. The drug has been developed in collaboration with Essex Bio-Technology, which is also headquartered in China, for the treatment of wet AMD [1].
Henlius’ single-arm, phase I clinical trial is being conducted in six to 12 patients with wet AMD and aims to evaluate the safety, tolerability, and pharmacokinetics of HLX04-O. It follows a series of earlier studies investigating the pharmacodynamics and pharmacokinetics, safety, toxicity, and immunogenicity of HLX04-O.
In September 2021, Henlius reported results from a phase III clinical trial of the bevacizumab copy biological HLX04 in patients with colorectal cancer. The results were presented at the Chinese Society of Clinical Oncology (CSCO) meeting 2021 and related to a study of 675 patients with metastatic colorectal cancer.
HLX04 was compared to reference bevacizumab (in combination with chemotherapy) in terms of efficacy, safety, and immunogenicity. Equivalent clinical efficacy was demonstrated in terms of overall survival, progression-free survival, objective response rate, time to response and duration of response. Safety and immunogenicity profiles were also similar between HLX04 and reference bevacizumab, leading Henlius to conclude that HLX04 could be a potential alternative treatment option for patients with colorectal cancer.
In related news, Innovent Biologics has reported that their bevacizumab biosimilar IBI305 could be used to treat hepatocellular carcinoma. In data published in The Lancet Oncology, Innovent reports the use of IBI305 in combination with sintilimab. Sintilimab (Tyvyt) is a monoclonal antibody inhibitor of PD-1, which can prevent the immune system from killing cancer cells. The trial combined the treatments in patients with unresectable, HBV-associated hepatocellular carcinoma.
The randomized, open-label, phase II/III study was conducted across 50 clinical sites in China. The results found significant benefits for overall survival and progression-free survival of IBI305 plus sintilimab, compared to sorafenib (Nexavar), a kinase inhibitor approved for the treatment of hepatocellular carcinoma. The safety profile of IBI305 plus sintilimab was also found to be acceptable, leading the authors to conclude that ‘this combination regimen could provide a novel treatment option for such patients’.
Innovent has also begun a phase II trial for an ipilimumab copy biological, for the treatment of patients with advanced cervical cancer [2].
Related articles
Challenges facing copy biologicals in China
Innovent makes deal for bevacizumab copy biological in Indonesia
China approves adalimumab copy biological HLX03
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View last week’s headline article: Los datos apoyan la intercambiabilidad de los biosimilares de la UE Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de la semana pasada: Los datos apoyan la intercambiabilidad de los biosimilares de la UE Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. GaBI Online - Generics and Biosimilars Initiative. Essex and Henlius collaborate to deliver bevacizumab biosimilar for ophthalmic diseases [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Nov 5]. Available from: www.gabionline.net/pharma-news/Essex-and-Henlius-collaborate-to-deliver-bevacizumab-biosimilar-for-ophthalmic-diseases
2. GaBI Online - Generics and Biosimilars Initiative. Innovent starts phase II trial for ipilimumab copy biological [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Nov 5]. Available from: www.gabionline.net/biosimilars/news/Innovent-starts-phase-II-trial-for-ipilimumab-copy-biological
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Source: The Lancet Oncology








 0
0










Post your comment