The development of biosimilar medicines presupposes a paradigm shift from the traditional model used with an innovative medicine. Meanwhile, for a new molecular entity, the evidence for its development is based on generating data confirming its safety and efficacy.
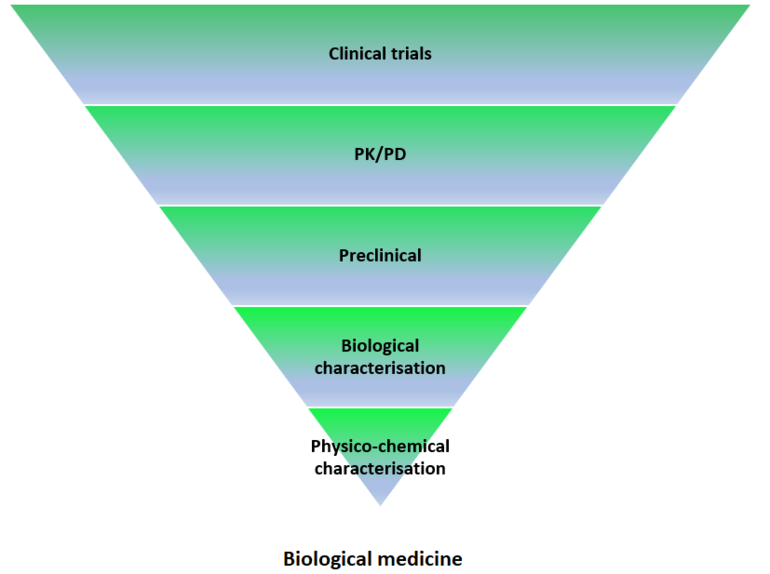
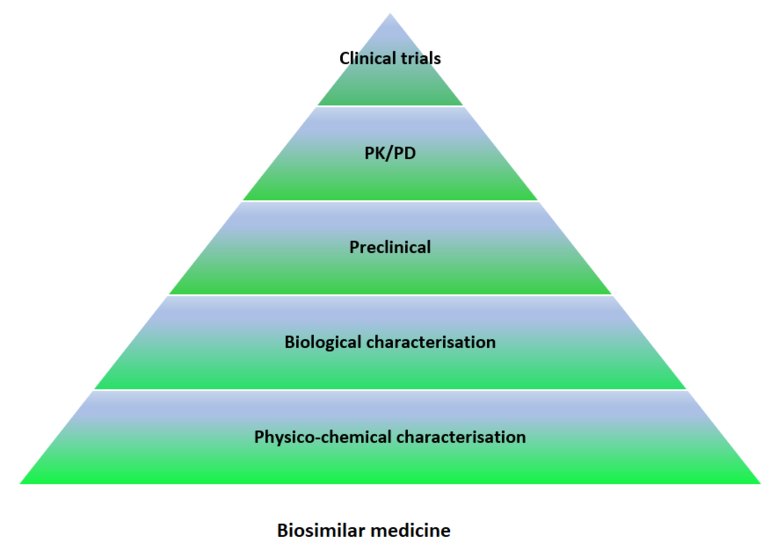
In the case of biosimilars, the main objective is the demonstration of similarity between the molecular structure, almost always protein, of the original drug and that of the proposed biosimilar, see Figure 1.
Figure 1: Data requirements for authorization of biological versus biosimilar medicine
In the development of biosimilar medicines, the following should be considered:
- the biosimilar must contain the same primary structure, i.e. amino acid sequence, and the same 3D structure (protein folding) as the reference drug
- a comprehensive and product-specific comparison of functional properties, pharmacokinetics, pharmacodynamics as well as clinical efficacy, safety and immunogenicity is performed
- for the finished product, both the biosimilar and the reference drug should have the same dosage and route of administration
- certain differences may be permitted if they do not affect safety and efficacy, e.g. differences in the formulation of the medicinal product, e.g. excipients; in the presentation and dispensing, e.g. type of injection device [1].
The comparability study is divided into well-established and product-specific sequential steps. In each of these, acceptance criteria are established to determine the degree of similarity between the biosimilar and biological product taken as reference. After each step, an assessment is made as to whether it can be continued or by contrary, whether it is necessary to require further preclinical or clinical studies. If at any step differences are considered substantial, or the comparison exercise is incomplete, the medicine cannot be considered biosimilar.
The comparability stages include:
- Analytical comparability: in vitro studies compare protein structure and biological function using sensitive techniques capable of detecting minimum differences with clinical relevance.
- Preclinical comparability: This includes pharmacodynamic studies, mainly in vitro, looking at binding and activation (or inhibition) of physiological targets and immediate physiological effects in cells. In vivo toxicology studies are only required in some very specific cases, e.g. when the biosimilar is produced by a new type of cell or organism, or when the formulation includes novel excipients not previously used.
- Clinical comparability: The aim of human studies is not to demonstrate safety and efficacy in patients, as these parameters have already been established for the reference drug product. Comparative clinical trials are used to confirm that both products contain highly similar active substances and that their quality, efficacy and safety are equivalent. The number and type of clinical trials required depends on many factors. These include the complexity of the molecule under study, the data obtained from previous phases of the comparability study, and the availability of validated clinical markers that can be correlated with efficacy [1].
Editor’s comment
Readers interested to learn more about the authorization of biosimilars are invited to visit www.gabi-journal.net to view the following manuscript published in GaBI Journal:
The EU regulatory network and emerging trends – a review of quality, safety and clinical development programmes
GaBI Journal is indexed in Embase, Scopus, Emerging Sources Citation Index and more.
Readers interested in contributing a research or perspective paper in Spanish and/or English on biosimilars to GaBI Journal – an independent, peer reviewed academic journal – please submit your manuscript here.
GaBI Journal Citation Impact
1.9 – CiteScore 2020 (calculated on 5 May 2021)
1.9 – CiteScoreTracker 2021 (Last updated on 4 August 2021)
Submit a manuscript to GaBI Journal
Related articles
Diferencias entre los medicamentos genéricos y los biosimilares
Position of CAEME on biological and biosimilar drugs in Argentina
| LATIN AMERICAN FORUM The brand-new section the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View this week’s headline article: México presenta nuevo decreto sobre regulación sanitaria Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de esta semana: México presenta nuevo decreto sobre regulación sanitaria Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Latin American Forum. Informe a colegas y amigos sobre esta nueva iniciativa.
|
Reference
1. Comisión Europea. Los biosimilares en la UE. Guía informativa para profesionales sanitarios.15/10/2018 [cited 2021 Aug 27]. Available from: https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information- guide-healthcare-professionals_es.pdf
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment