The rate of uptake of biosimilars has accelerated as stakeholders have become increasingly comfortable with these products [1]. However, the adoption of biosimilars is lower in regions experiencing low social and governmental trust [2, 3].
In a presentation by Mr Aurelio Arias of IQVIA, the data showed that the biosimilar market averages 40% after 12 months of launch and is likely to reach 60% after 24 months. The adoption of biosimilars has quickened as stakeholders become increasingly comfortable with the comparable biologicals.
Biosimilar uptake rates in Europe
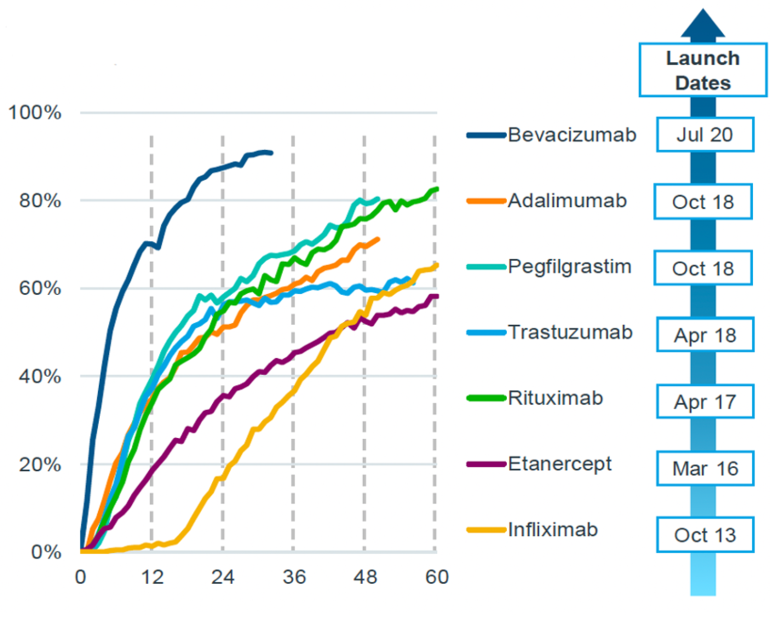
The uptake rates of biosimilars have been progressively rising from October 2013 to July 2020, as shown in Figure 1. This figure displays the months since the launch of the biosimilar and the percentage of treatment days filled by biosimilars. It demonstrates that the rate of uptake increased over time for the biosimilars of bevacizumab, adalimumab, pegfilgrastim, trastuzumab, rituximab, etanercept and infliximab.
Figure 1: Biosimilar adoption rates in Europe
Source: IQVIA
For instance, in the case of the biosimilar for bevacizumab, the percentage of treatment days filled by biosimilars was approximately 20% six months after its launch. This percentage then increased to more than 80% within 24 months of the launch.
Biosimilar uptake rates in the UK
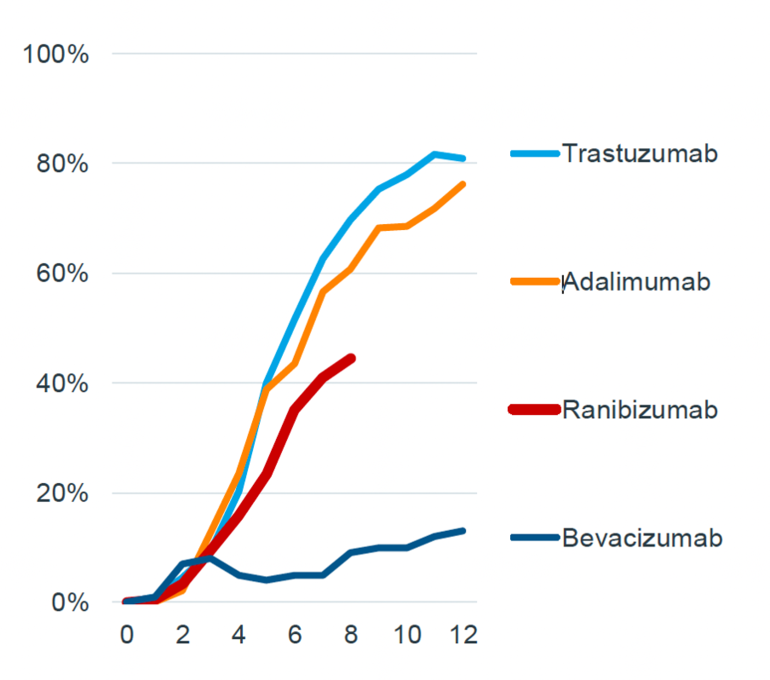
The UK has faster biosimilar uptake rates within the first 12 months of launch compared to Europe.
Taking the example of biosimilar trastuzumab, the percentage of treatment days filled by biosimilars exceeded 80% within 10 months of its launch in the UK, whereas it took 12 months to reach 40% in Europe.
Figure 2: Biosimilar adoption rates in the UK
Source: IQVIA
This trend of increased uptake of biosimilars is likely to continue in Europe in the future, and high-value biologicals are expected to have biosimilar alternatives.
Omnitrope (somatropin) was the first biosimilar approved in the EU in 2006. To date, the European Medicines Agency has recommended the approval of 96 biosimilars [4].
Europe has been at the forefront of encouraging the use of biosimilars [5], and now the US uptake is also starting to catch up, with its adoption of biosimilars rivalling that of Europe [6].
Related articles
Emerging disparities in market concentration among biosimilars
Biosimilar development targets limited range of biologicals
Biosimilars in Southern European hospital markets: barriers and determinants of uptake
References
1. Arias A. Filling the Biosimilar Void. 19th Biosimilars Medicines Conference; 2023 May 25-26: Amsterdam, The Netherlands. Medicines for Europe.
2. Robinson JC. Social trust and regional variation in the adoption of biosimilars in Italy and Germany. Generics and Biosimilars Initiative Journal (GaBI Journal). 2022;11(3):87-8. doi:10.5639/gabij.2022.1103.015
3. GaBI Online - Generics and Biosimilars Initiative. Low biosimilar uptake in regions of low social and political trust[www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Sep 8]. Available from: www.gabionline.net/biosimilars/research/low-biosimilar-uptake-in-regions-of-low-social-and-political-trust
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Aug 28]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
5. GaBI Online - Generics and Biosimilars Initiative. The US needs to learn from Europe to increasing access to biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Aug 28]. Available from:
www.gabionline.net/biosimilars/research/the-us-needs-to-learn-from-europe-to-increasing-access-to-biosimilars
6. Reilly MS, Schneider PJ. Key factors for successful uptake of biosimilars: Europe and the US. Generics and Biosimilars Initiative Journal (GaBI Journal). 2022;11(3):112-24. doi:10.5639/gabij.2022.1103.018
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment