Poland-based Mabion announced on 1 June 2018 that its candidate rituximab biosimilar (MabionCD20) had been accepted for regulatory review by the European Medicines Agency (EMA).
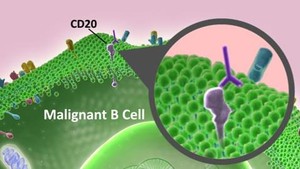
Rituximab is a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of immune system B cells. Rituximab destroys B cells and is therefore used to treat diseases that are characterized by excessive number of B cells, overactive B cells or dysfunctional B cells. This includes many lymphomas, leukaemias, transplant rejection and autoimmune disorders.
The product is a proposed biosimilar to Roche/Genentech’s MabThera/Rituxan (rituximab), which had worldwide sales of CHF 7.3 billion (Euros 6.4 billion) in 2016, before the advent of biosimilars. The patents on MabThera/Rituxan expired in the US in September 2016 and in Europe in February 2013 [1].
Mabion signed a development and commercialization agreement with Mylan Ireland (a subsidiary of Mylan) in November 2016, giving Mylan the right to commercialize the rituximab biosimilar in the EU and non-EU Balkan states [2].
The submission includes results of the clinical trial carried out on MabionCD20 and conducted in rheumatoid arthritis and non-Hodgkin’s lymphoma patients. According to Mabion, this trial ‘confirmed that the drug is equivalent to its reference drug, MabThera’.
There are already six rituximab biosimilars approved in Europe. South Korean biotechnology company Celltrion received approval for Truxima (CT‑P10) in February 2017 [3] and received approval for Blitzima, Ritemvia and Rituzena (previously Tuxella) in July 2017 [4]. Sandoz received approval for Rixathon (GP2013) and Riximyo (GP2013) in June 2017 [5].
Mabion also states that it has arranged a meeting with the US Food and Drug Administration (FDA) at which it intends to finalize ‘the most optimal pathway towards registering MabionCD20 in the USA’.
Genentech announced on 7 June 2018 that it had received FDA approval for Rituxan for pemphigus vulgaris, a rare, serious, potentially life-threatening condition characterized by progressive painful blistering of the skin and mucous membranes. This is the first FDA-approved treatment for moderate to severe pemphigus vulgaris in more than 60 years. This extends Rituxan’s approved treatments to include four autoimmune indications: chronic B-cell lymphocytic leukaemia, Non-Hodgkin’s lymphoma, pemphigus vulgaris and rheumatoid arthritis.
This news from Genentech could make life difficult for biosimilars makers who are already busy with the clinical plans for their rituximab biosimilars or have already submitted applications to FDA. Having different indications approved for biosimilars and the originator biological could potentially make it even more difficult to gain the holy grail of interchangeability for rituximab biosimilars.
Related articles
Celltrion resubmits biosimilar rituximab to FDA
FDA rejects Celltrion/Teva’s rituximab and trastuzumab biosimilars
References
1. Derbyshire M. Patent expiry dates for biologicals: 2017 update. Generics and Biosimilars Initiative Journal (GaBI Journal). 2018;7(1):29-34. doi:10.5639/gabij.2018.0701.007
2. GaBI Online - Generics and Biosimilars Initiative. Mabion signs agreement with Mylan for rituximab biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jul 20]. Available from: www.gabionline.net/Pharma-News/Mabion-signs-agreement-with-Mylan-for-rituximab-biosimilar
3. GaBI Online - Generics and Biosimilars Initiative. EC approval for first cancer biosimilar Truxima [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jul 20]. Available from: www.gabionline.net/Biosimilars/News/EC-approval-for-first-cancer-biosimilar-Truxima
4. GaBI Online - Generics and Biosimilars Initiative. EC approval for three rituximab biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jul 20]. Available from: www.gabionline.net/Biosimilars/News/EC-approval-for-three-rituximab-biosimilars
5. GaBI Online - Generics and Biosimilars Initiative. EC approval for rituximab biosimilar Rixathon [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2018 Jul 20]. Available from: www.gabionline.net/Biosimilars/News/EC-approval-for-rituximab-biosimilar-Rixathon
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2018 Pro Pharma Communications International. All Rights Reserved.
Source: Genentech, Mabion








 0
0










Post your comment