Biosimilars/Research
|
Posted 13/07/2018
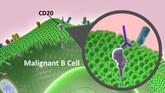
In 2015, the US Food and Drug Administration (FDA) approved its first biosimilar, filgrastim-sndz, a biosimilar of the granulocyte colony-stimulating factor filgrastim. Since then, FDA has approved four additional biosimilar tumour necrosis factor α inhibitors, and in May 2017, the Oncology Drug Advisory Committee voted in favour of approval of an epoetin alfa biosimilar. Three biosimilar monoclonal antibodies (mAbs) have been approved in the US. Although their indications are for chronic inflammatory diseases, according to authors Robert Rifkin and Susan Peck, oncologists should become familiar with these agents, because they may need to administer these drugs for patients who have concurrent chronic inflammatory conditions [1].