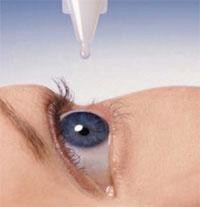
On 2 February 2022, the US Food and Drug Administration (FDA) approved the first generic of Restasis (cyclosporine ophthalmic emulsion).
The newly approved cyclosporine generic is sponsored by Mylan Pharmaceuticals Inc and has been approved as 0.05% single-use vials (eye drops). Restasis is used in the treatment of keratoconjunctivitis sicca, or dry eye, and acts to increase tear production in patients whose tear production is suppressed due to ocular inflammation.
This comes after several years of controversy over Restasis generics. Initially, the originator company Allergen, requested that FDA revise its guidance on cyclosporines, arguing that generic versions of Restasis be tested in humans before approval, not just in a laboratory [1]. Then, in October 2017, a federal judge in Texas invalidated four key patents for Allergen’s Restasis following the originators company’s deal that transferred the patent rights to the Saint Regis Mohawk Tribe [2, 3]. In addition, Israel’s Teva Pharmaceuticals launched unsuccessful lawsuits in the US against FDA over marketing exclusivity of Restasis [4, 5].
Sally Choe, PhD, Director of the Office of Generic Drugs in FDA’s Center for Drug Evaluation and Research, said that the ‘approval reflects the FDA’s continued commitment to advancing patient access to lower-cost, high-quality generic drug products that are as safe and effective as their brand name counterparts’, and that its FDA’s major focus on ‘supporting development and expanding opportunities to bring complex generic drugs to the market’.
The development of complex generics may be more difficult due to, for example, their complex active ingredient formulation or route of delivery. Thus, many complex drugs lack generics competition. The FDA has taken a multifaceted approach to encourage development of complex generics through the Generic Drug User Fee Amendments (GDUFA) program.
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View this week’s headline article: Nomenclatura de biológicos y biosimilares en Brasil Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de esta semana: Nomenclatura de biológicos y biosimilares en Brasil !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
Related articles
FDA approves schizophrenia and anti-allergy drugs
US generic approvals for Acrux and Cipla
FDA approval for schizophrenia and HIV generics from Zydus Cadila and Lupin
References
1. GaBI Online - Generics and Biosimilars Initiative. Allergan objects to Restasis generics being accepted without human trials [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 Feb 25]. Available from: www.gabionline.net/generics/news/Allergan-objects-to-Restasis-generics-being-accepted-without-human-trials
2. GaBI Online - Generics and Biosimilars Initiative. Texan judge invalidates Restasis patents [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 Feb 25]. Available from: www.gabionline.net/generics/general/Texan-judge-invalidates-Restasis-patents
3. GaBI Online - Generics and Biosimilars Initiative. US tribal patent deal could prevent generics [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 Feb 25]. Available from: www.gabionline.net/generics/general/US-tribal-patent-deal-could-prevent-generics
4. GaBI Online - Generics and Biosimilars Initiative. US Court rejects Teva’s lawsuit over generic Restasis [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2022 Feb 25]. Available from: www.gabionline.net/generics/general/US-Court-rejects-Teva-s-lawsuit-over-generic-Restasis
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2022 Pro Pharma Communications International. All Rights Reserved.








 0
0






Post your comment