Costs and risk reduction are facilitating product development of biosimilars [1].
Experience and outsourcing have yielded substantial savings in development costs and the time taken to develop a biosimilar.
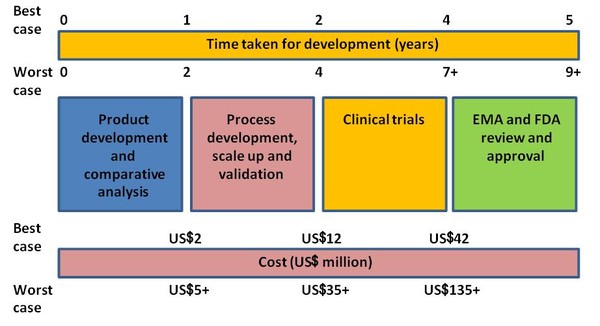
There are four stages in the development of a biosimilar: 1) product development and comparative analysis; 2) process development, scale up and validation; 3) clinical trials; 4) Regulatory (EMA and FDA) review and approval. All stages come with varying requirements and take varying amounts of time, all of which contributes to the overall cost of developing a biosimilar, see Figure 1.
1. Product development and comparative analysis
During this stage, it is necessary to create cells that reproduce the protein of interest and validate their stability. The product must also demonstrate that it is biosimilar to the originator drug.
2. Process development scale up and validation
During this stage, scale up of manufacturing needs to be carried out, along with improvement of yields. Processes to ensure good manufacturing practices need to be established and reproducibility of the manufacturing process needs to be demonstrated.
3. Clinical trials
Clinical trials will be required for almost all products in order to demonstrate bioequivalence.
4. Regulatory (EMA and FDA) review and approval
In Europe, the regulatory frameworks for biosimilars are largely established, with the EMA having issued both general and product specific guidelines. The EMA also operates a system of biosimilar fees, with the fee for a biosimilar being around 64% of the fee for a new biological, reflecting the lower workload [2].
In the US, the FDA is also discussing biosimilar user fees, however, it does not yet have a practical pathway with guidance defined by the FDA. The legal pathway for biosimilars was signed into law on 23 March 2010 by President Barack Obama as part of the Biologics Price Competition and Innovation [BPCI] Act.
For the completion of all four stages in the development of a biosimilar timescales of five to nine years or more have been suggested. Similarly, on the subject of cost, ranges of US$42 to 135 million, excluding regulatory fees, has been put forward as reasonable for the development of a biosimilar, see Figure 1.
Figure 1: Costs and timescales for biosimilar development
Source: Bernstein Research
For the antibodies industry, Bernstein research suggests that US$20-40 million should cover development costs ‘from sequence to first in man’ with a timescale of 5-9 years being not unreasonable.
In the meantime, many Big Pharmas are fighting the expected wave of biosimilars with biobetters. Biobetters will almost always precede biosimilars [1] and, since biobetters will also have patent-protection, can take some of the sting out of patent losses on the originator biological products.
Biobetters are improvements to originator biological molecules, whereas biosimilars are structural imitations of the originator. While biosimilars promise the same effect at a reduced price, a biobetter will possess some molecular or chemical modification that constitutes an improvement over the originator drug and its biosimilar competitors.
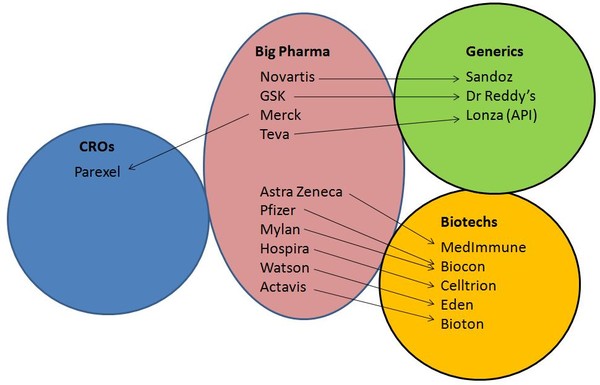
Many major players in the pharmaceutical industry—both Big Pharma and generics companies alike—are already showing an interest in the biosimilars market, see Figure 2. Actavis and Bioton announced an agreement back in September 2010, while Merck has entered into an alliance with contract research organisation (CRO) Parexel to provide biosimilars to Merck BioVentures. Even non-pharma are getting in on the act, with electronics giant Samsung signing a deal to produce both originator biotech products and biosimilars with CRO Quintiles.
Meanwhile, Sandoz, the generics unit for Novartis, has already proved itself to be a major player in the biosimilars market, being the only manufacturer so far to have more than two biosimilars on the market.
Figure 2: Partnerships for biosimilar development
Source: Bernstein Research
Lower costs are the driving force for many players in the pharmaceutical industry to develop biosimilars. Mr Ronny Gal believes that eventually the biosimilar field is likely to be more populated than the one to two players some would predict, with five biosimilar competitors for a single biological drug being not unusual.
Related articles
Timing of the launch of biosimilars in Europe
How cheap will biosimilars need to be
The market for biosimilars
Biosimilar regulatory issues
Manufacturing of biosimilars
Challenges ahead for biosimilar development
EGA meeting London 2011: biosimilars competitiveness in the EU
References
1. Gal R. Biosimilar development is progressing, but bigger challenges are ahead. 9th EGA International Symposium on Biosimilar Medicines; 2011 Apr 14; London, UK.
2. GaBI Online - Generics and Biosimilars Initiative. EMA and FDA to collaborate on biosimilars. [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2011 July 5]. Available from: www.gabionline.net/Biosimilars/News/EMA-and-FDA-to-collaborate-on-biosimilars








 0
0











Post your comment