Author: Fiona M Greer, BSc (Hons), MSc, PhD, Independent Consultant
Abstract
Debate continues on the requirement for different types of data to demonstrate biosimilarity, with some regulators expressing expectations of a future without large expensive comparative efficacy studies. Proposed changes of regulatory emphasis reinforce the importance of choosing orthogonal analytical techniques to interrogate quality attributes (QA) when assessing biosimilarity.
Keywords: Biosimilar testing, biosimilarity, characterization techniques, orthogonality, regulatory requirements
Introduction
Evolution of biosimilar guidelines
For some time now, a hot topic of discussion has concerned the types of data, non-clinical and clinical, required for demonstration of biosimilarity and the ability of analytical characterization to provide an accurate understanding of originator and biosimilar molecules, in turn arguably reducing the need for large phase III clinical trials.
In April 2020, international industry experts reviewed public information on both EU and US biosimilar approvals since 2006, concluding that with current analytical methods, a tailor-made development for biosimilars need not routinely require comparative efficacy trials [1]. In September 2020, a position paper from experienced experts working at the UK Medicines and Healthcare products Regulatory Agency (MHRA) also examined this issue, reviewing 20 EU biosimilar applications covering six complex reference products [2]. They also questioned whether clinical efficacy trials are still necessary, given their limitations as an effective discriminating tool and argued that, excluding exceptional cases, biosimilarity can be demonstrated by analytical testing and pharmacokinetic (PK) trials.
Shortly afterwards MHRA proposed new draft guidance for biosimilar licensing, including biosimilar testing [3] which, although based on current European Medicines Agency’s (EMA) guidance, diverges particularly in the requirement for a comparative efficacy trial. It signals a shift away from assessments involving clinical comparability, in favour of robust and extensive physicochemical and biological testing together with PK studies. In essence, the MHRA supports the concept of in-depth scientific knowledge of the reference product (RP) gained using state-of-the-art analytical techniques to interrogate structure and biological activity then subsequently used in comparative assessments of the biosimilar, together with a pivotal comparative PK trial with no requirement for comparative efficacy/safety trials. The paper concludes that ‘extensive comparative analytical studies, together with an abbreviated clinical package …, is sufficient to assess biosimilarity in most cases’. We await publication of comments obtained during the consultation period and the resulting updated guidance.
Importance of orthogonality in biosimilarity assessment
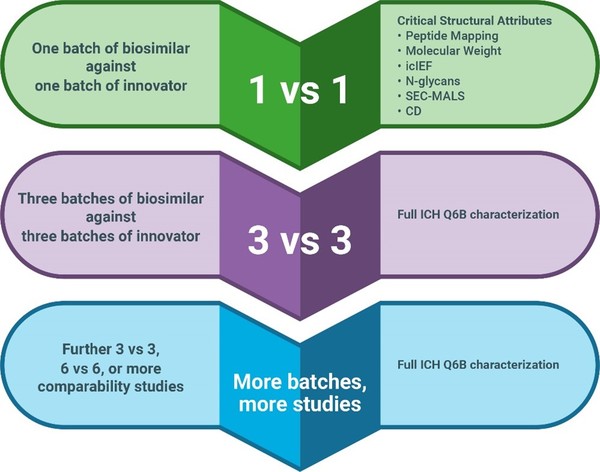
The central premise in any case for omitting a comparative efficacy trial is that extensive quality data should be generated, highlighting the importance of detecting and defining ranges of critical quality attributes (CQA) of the molecules at both an analytical and in vitro functional level. The big question being – what types of analytical, non-clinical data are most appropriate to support demonstration of biosimilarity? For analytical scientists, the spotlight is turned upon the choice of analytical methods to perform both the detailed structural interrogation of the RP and the comparability exercise with the biosimilar such as outlined in Figure 1. As regulatory authorities have extensive experience with modern analytical characterization techniques, they certainly expect the ‘big guns’ to be used. The draft MHRA guidance, for example, makes this clear, stating: ‘… comprehensive analysis of the proposed biosimilar and RP, using state-of-the-art methods with suitable sensitivity and orthogonal methods to determine not only similarities but also potential differences.’
Figure 1: Structural comparability studies performed over time to obtain statistically significant data across a range of biosimilar and originator batches. A bridging or reference sample can be used to allow comparability assessments to be made between different studies
The US Food and Drug Administration (FDA), EMA and MHRA all state in their current and draft guidance documents that orthogonal methods should be used for comparability studies to give strength to the analytical data package. A multitude of methods can be chosen for structural characterization but the key, however, is knowing how to apply them in the most meaningful way so that conclusions from one particular technique can be matched (if possible) to data obtained from another. The table below gives examples of orthogonal techniques and their application to specific aspects of structure. Some approaches can be broadly applied, being common to all (glyco)proteins, however, some molecules will have unique aspects of structure for which specific applications will be required, such as the extent of C-terminal lysine on the heavy chains of monoclonal antibodies.
| Table 1: Examples of orthogonal techniques and their application to specific aspects of structure
|
| Technique
|
Aspect
|
Orthogonal technique(s)
|
Orthogonal parameter
|
| Primary structure
|
| MS Peptide mapping
|
Mass and fragment ion-based id of heavy chain C-term peptide(s)
|
Imaging capillary isoelectric focusing
|
Charge-based separation of mAbs with/without heavy chain(s) containing C-term Lys
|
| MS Peptide mapping
|
Mass and fragment ion-based id of deamidated peptides
|
Imaging capillary isoelectric focusing
|
Charge-based separation of mAbs
|
| MS Peptide mapping
|
Disulfide bridges
|
HOS techniques such as CD, NMR
|
Assessment of overall protein structure at the secondary and tertiary levels
|
| MS Peptide mapping
|
Assessment of N- and C-termini
|
MS intact mass analysis, Edman sequencing (N-terminus only)
|
Mass determination of intact species. Sequential removal and id of N-terminal amino acids
|
| On-line LC-MS of fluorescently tagged released N-glycans
|
N-Glycan analysis
|
MALDI-MS, peptide mapping
|
Assessment of alternatively derivatized released N-glycans. Assessment of glycopeptide mass profiles
|
| Secondary structure
|
| Circular Dichroism
|
Spectroscopic assessment of HOS (secondary/tertiary)
|
Fourier Transform Infrared spectroscopy, IF/EF, NMR
|
Alternative mechanisms for assessing HOS through different forms of spectroscopy
|
| Aggregation
|
| Size exclusion chromatography with Multi Angle Laserlight Scattering
|
Assessment of aggregation through chromatographic separation with light scattering assessment of aggregate size
|
Sedimentation Velocity Analytical Ultracentrifugation
|
Separation of aggregates through interaction with a centrifugal field and UV and interference optics measurement
|
| Host Cell Proteins (HCPs)
|
| ELISA
|
Assessment of HCPs through gel-based separation and immunochemical detection
|
Mass Spectrometry
|
Assessment of protein fragments with database interrogation for protein id. Quantitation of HCPs through proteomic type analyses
|
| CD: circular dichroism; HOS: higher order structure; id: identity; LC-MS: liquid chromatography–mass spectrometry; mAbs: monoclonal antibodies; MS: mass spectrometry; NMR: nuclear magnetic resonance; UV: ultraviolet; MALDI: matrix-assisted laser desorption/ionization.
|
Indeed, the challenges of demonstrating biosimilarity have driven analytical method development with emerging novel techniques, improvements in older ‘classical’ methods and a greater appreciation of methods which link structure with biological activity or predict how the molecule might interact within the biological system, for example, HDX-MS. Previous gold-standard research techniques such as Protein NMR and X-Ray crystallography are also being utilized more.
Dr Andrew Reason, CEO and MD of BioPharmaSpec, a global CRO offering structural and physicochemical characterization services, commented ‘An emphasis on analytical characterization highlights the importance of using the correct combination of analytical techniques, coupled with experienced interpretation of the findings to assess observed differences for further study using appropriate discriminating methods’.
Essentially, regulators are looking for multiple orthogonal assessments to build a total profile of the molecule. This use of orthogonality in experimental design strengthens the value of the data beyond what each individual characterization technique could achieve.
References
1. Schiestl M, Ranganna G, Watson K, et al. The path towards a tailored clinical biosimilar development. BioDrugs. 2020;34(3):297-306.
2. Bielsky M-C, Cook A, Wallington A, et al. Streamlined approval of biosimilars: moving on from the confirmatory efficacy trial. Drug Discov Today. 2020;25(11):1910-8.
3. Gov. UK. Consultation document: MHRA guidance on the licensing of biosimilar products. 7 October 2020 [homepage on the Internet]. [cited 2021 Mar 5]. Available from: https://www.gov.uk/government/consultations/mhra-draft-guidance-on-the-licensing-of-biosimilar-products/consultation-document-mhra-guidance-on-the-licensing-of-biosimilar-products








 0
0







Post your comment