Author: Jennifer Campbell; Christopher Gillespie, PhD; Michael Phillips, PhD
The biosimilars market continues to expand, with forecasts for significant market penetration, as already evidenced in regions where biosimilars have been on the market for years [1]. The premise of biosimilars is affordable health care, with the hope of expanding accessibility to populations previously not served by biological medicines [2]. In this endeavour, developers of biosimilars are targeting dramatically reduced costs of goods (CoGs). They must compete with originators who manufacture drugs in large stainless steel facilities, with the benefit of economies of scale. Most biosimilar molecules will be manufactured at scales at or below 2,000 L and primarily leverage single-use platforms [3]. This presents a conundrum for cost reduction as the cost of goods for the 2,000 L single-use solution is often considerably higher than the 10,000 stainless steel solution. In order for biosimilars to effectively compete on economics, they will need to take advantage of new technologies that offer both performance and cost advantages.
In this article we will explore methods to reduce the costs of goods for monoclonal antibody (mAb) manufacturing through process intensification. Holistic process modelling of the traditional mAb template reveals that several opportunities exist for decreasing COGs [4]. From an upstream perspective, the key drivers for decreasing COGs are through improving bioreactor capacity utilization – a combination of cellline engineering and cell culture media optimization. From a downstream perspective, the affinity capture step and the polishing/purification steps are often the most cost and time intensive unit operations. Our focus in this article will be to highlight downstream opportunities for increasing specific productivity and decreasing COGs.
Due to the combination of low resin utilization and sequential operation of process steps, batch chromatography has several opportunities for significant process intensification. Bottlenecks that limit productivity may result from batch column size and media underutilization, large buffer consumption, and long processing times due to column cycling requirements. The most common approaches for increasing the productivity of batch chromatography include operating at reduced residence times (increased flow rates), increasing the inlet concentration to minimize productloading time, and transitioning to continuous multi-column chromatography (CMC).
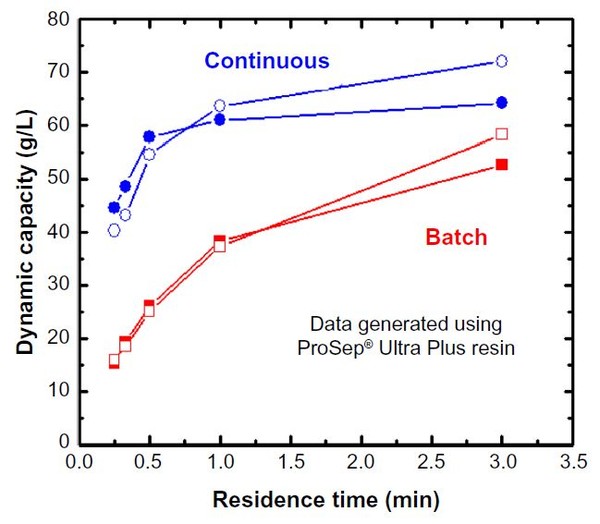
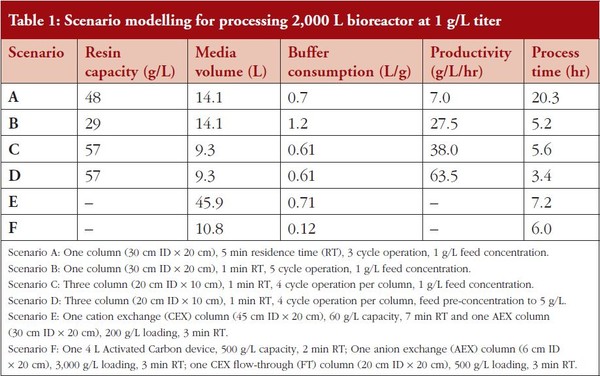
Depending upon the specifics of the chromatography media, decreasing the residence time often results in a significant decrease in effective dynamic binding capacity (DBC). Figure 1 shows the mAb05 (IgG1, pI ∼8.3) dynamic capacity on ProSep® Ultra Plus – a rigid Protein A affinity media. For a single-column process, reducing the residence time (RT) from 5 mins to 1 min reduces the effective loading capacity from approximately 48 g/L to 29 g/L (loading capacity is 80% of dynamic capacity), a decrease of approximately 40%. Process modelling for this system calculates that the specific productivity of this unit operation will increase from 7 g/L/hr (5 min RT) to 27.5 g/L/hr (1 min RT) due primarily to a decrease in processing time (20.3 hr to 5.2 hr). The 5 min and 1 min residence time results are summarized in Table 1 as Scenarios A and B, respectively. Although feasible, decreasing the residence time is not the preferred intensification method as the productivity improvements come at a cost of increased buffer consumption (70% increase) and less efficient resin utilization (60% more cycles that impact resin lifetime). Both of these drawbacks combine to effectively increase the total COGs. CMC operation typically increases resin utilization from approximately 70% in batch mode to upwards of 90%. As seen in Figure 1, for highly effi cient, incompressible resins such as ProSep® Ultra Plus, CMC enables high capacity operation, even at residence times as low as 0.5 min. These high capacities at low residence times can be obtained with ProSep® Ultra Plus resin due to the rigid controlled pore glass base matrix with large pores, enabling high mass transfer properties. Compared to our base case of a 5 min residence time run in batch mode, running ProSep® Ultra Plus resin at a 1 min residence time in CMC mode increases load ing capacity from 48 g/L to 57 g/L, resulting in a productivity increase from 7 g/L/hr to 38 g/L/hr, a reduction in process time from 20.3 hr to 5.6 hr, a 13% reduction in buffer volumes, and a more efficient utilization of the affinity media, thereby decreasing overall COGs.
Figure 1: Dynamic capacity as a function of residence time for batch and continuous mode
Table 1: Scenario modelling for processing 2,000 L bioreactor at 1 g/L titer
The CMC data is summarized in Table 1 as Scenario C. Note that the DBC stated for the CMC mode is the effective DBC for the primary load column. As seen in Figure 1, due to the rigid base matrix and high mass transfer properties of ProSep® Ultra Plus resin, productivity could be even further enhanced by operating at a residence time of 0.5 min.
A third option for process intensifi cation of the affinity capture step is preconcentration of the feed using single-pass tangential flow filtration (SPTFF). SPTFF is an effective in-line method for preconcentrating feed prior to loading onto Protein A. Pre-concentration shows benefits in batch and CMC mode. In batch mode, with a 5 min RT, increasing load concentration from 1 g/L to 5 g/L resulted in a 90% productivity increase from 7 g/L/hr to 13.3 g/L/hr, with process time reduced from 20.3 hr to 10.7 hr. The productivity increase is a direct result of the decreased loading time. In CMC mode, with a 1 min RT, increasing load concentration from 1 g/L to 5 g/L resulted in a 67% productivity increase from 38 g/L/hr to 63.5 g/L/hr, with process time reduced from 5.6 hr to 3.4 hr. The high titer CMC results are highlighted in Table 1 as Scenario D. Clearly, CMC benefits from rapid loading. For high titer processes, additional increases in productivity can be achieved by utilizing more columns.
These options offer time and cost savings for the affinity capture operation. Depending upon existing bottlenecks or facility constraints, different options may be advantageous in existing or new processes. For polishing and purification, the traditional mAb processing train typically consists of a bind-elute cation exchange unit operation followed by a flow-through anion exchange operation. Similar to the capture unit operation, typical means for increasing productivity and reducing process economics include operating at higher flow rates, employing higher capacity media, employing single pass filtration to pre-concentrate the feed, and transitioning to CMC. Many of these options have been discussed above and the analysis will not be repeated.
Another option for improving the productivity of the polishing and purification unit operations is to move to an all flowthrough process. For mAb processing, it has been demonstrated that an all flowthrough polishing train employing existing and newly developed chemistries is capable of achieving comparable reduction of host cell proteins (HCP), DNA, aggregate, leached Protein A and virus, as well as comparable charge variant and glycoform profile compared to the traditional purification template while being able to achieve an 85% yield with minimal product dilution [5]. This flow-through solution is comprised of four separate flow-through technologies; a Millistak+® Carbon Pod (AC) operated at pH 7 and loaded to 500 g/L, an anion exchange resin (AEX) such as Eshmuno® Q operated at pH 7 and loaded to 3,000 g/L, a newly developed cation exchange resin (CEX) designed for efficient aggregate removal operated at pH 5 and loaded to 1,000 g/L, and a Viresolve® Pro virus filter operated at pH 5 and loaded to > 3,500 g/m2.
Using the above information, process modelling calculations were conducted to compare the performance of the flowthrough purification train to that of the traditional cation exchange bind-elute (CEX BE) and AEX fl ow-through (AEX FT) polishing train, neglecting the contribution from the virus filter, as it was assumed that the performance would be the same in each purification template. For the base case of polishing a post-Protein A pool from a 2,000 L, 1 g/L bioreactor, process modelling calculations indicate that 45.9 L of resin would be required to effect the separation – 31.8 L of CEX media at a loading capacity of 60 g/L and 14.1 L of AEX media at a loading of approximately 200 g/L. This separation could be achieved in 7.2 hrs with a total buffer consumption of 0.71 L/g of purified mAb. To achieve these results, it was assumed that a 2X dilution of the CEX elution pool was required prior to loading onto the AEX flow-through operation. These results are summarized in Table 1 as Scenario E.
A similar process modelling analysis was conducted for the new integrated flowthrough polishing train. Utilizing the loading described above, it was calculated that the polishing operation could be achieved in only six hours and would require 10.8 L of resin, a 75% reduction compared to the traditional template. In addition, buffer consumption would only be 0.12 L/g of purifi ed mAb, an 83% reduction compared to the traditional template. These results are summarized in Table 1 as Scenario F.
From the above analysis, it is clear that the new flow-through polishing train offers significant advantages compared to the traditional purification template. In addition to significant resin and buffer savings, Biosolve process modelling has shown that additional benefits may include savings in capital expenditure (55% reduction), less product dilution (20% compared to 100%), reduction in footprint (32% reduction), and a reduction in COGs (∼50% reduction).These advantages are primarily driven by the lower resin volume requirements that enable the use of single-use technologies, the lower buffer volume requirements driving down the required buffer tank volumes, and the ability of these flowthrough technologies to be integrated into a single flow-through operation utilizing a single skid, ultimately driving down the need for intermediate hold tanks and process skids. An overall 50% reduction in COGs will help enable the potential for biosimilars to achieve their reduced cost targets for affordable health care.
Finally, another less tangible advantage associated with the flow-through polishing template is reduced labour requirements. In many emerging regions, biosimilar developers are facing the challenge of sourcing skilled labour. The reduction in the complexity and duration of manufacturing processes will help alleviate this challenge. Assuming the use of CMC, combined with the new flow-through process, can achieve a 50% reduction in process time, less labour hours are required; hence less operators are needed in the facility. The reduction of complex bind-elute operations to simpler flow-through operations may also reduce the chance of operator error in processing.
In summary, it has been shown that process intensification in the capture step as well as moving to an all flow-through polishing mAb template can have significant advantages while still meeting purification targets. The next generation mAb process platform developed and tested has shown excellent results for product quality profile, impurity removal, process yield and process economics. It offers many advantages over traditional mAb platforms, including reductions in process time, manufacturing footprint, CapEx, and process economics, as well as water and buffer needs.
Funding sources
This work was produced in-house at Millipore SAS.
This paper is sponsored by Millipore SAS.
Authors
Jennifer Campbell
Director WorldWide Biosimilars Market,
Millipore SAS, 39 Route Industrielle de la
Hardt, Molsheim FR-67120, France
Christopher Gillespie, PhD
Manager, Next Generation Downstream
BioProcessing, EMD Millipore Corporation,
80 Ashby Road, Bedford, Massachusetts,
01730, USA
Michael Phillips, PhD
Director, Next Generation BioProcessing,EMD Millipore Corporation, 80 Ashby Road,
Bedford, Massachusetts, 01730, USA
References
1. IMS Institute for Healthcare Informatics. Aitken M. Delivering on the potential of biosimilar medicines. the role of functioning competitive markets. March 2016 [homepage on the Internet]. [cited 2017 Feb 7]. Available from: http://www.imshealth.com/files/web/IMSH%20Institute/Healthcare%20Briefs/Documents/IMS_Institute_Biosimilar_Brief_March_2016.pdf
2. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefi ts. 2013;6(8):469-78.
3. Campbell J, Ribault S. Single-use technology enables flexible factories. BioProcess International. 15 June 2016.
4. Xenopoulos A. A new, integrated, continuous purification process template for monoclonal antibodies: process modeling and cost of goods studies. J Biotechnol. 2015;213:42-53.
5. Campbell J. Process intensifi cation for mab bioprocessing. BioProcessing Asia 2nd International Conference; 5–8 December 2016; Phuket, Thailand. Available from: http://www.bioprocessingasia.net/
Copyright © 2017 Pro Pharma Communications International








 0
0







Post your comment