According to a report entitled ‘The role of biosimilars in health’ by Professor Dr Claudio Tafla, a physician specializing in emergency medicine from the Pontifical Catholic University of São Paulo in Brazil, biological medicines consume around 40% of the federal public budget for the purchase of medicines in pharmaceutical assistance in the Unified Health System (Sistema Único de Salud, SUS), with rheumatoid arthritis being responsible for the greatest consumption of these resources [1].
Some of the most expensive drugs in the world (lomitapide, ataluren, idursulfase, eculizumab, galsulfase, beta-galactosidase, elosulfase, alpha-glucosidase, metreleptin and alpha-galactosidase), most of which are used to treat rare diseases (Duchenne muscular dystrophy, mucopolysaccharidosis, haemolytic-uraemic syndrome (HUS), hereditary angioedema, Pompe disease, Fabry disease and, as exceptions in this group, diabetes and various types of dyslipidaemia), accounted for an impact of R$965.3 million in 2017, for only 1,334 patients.
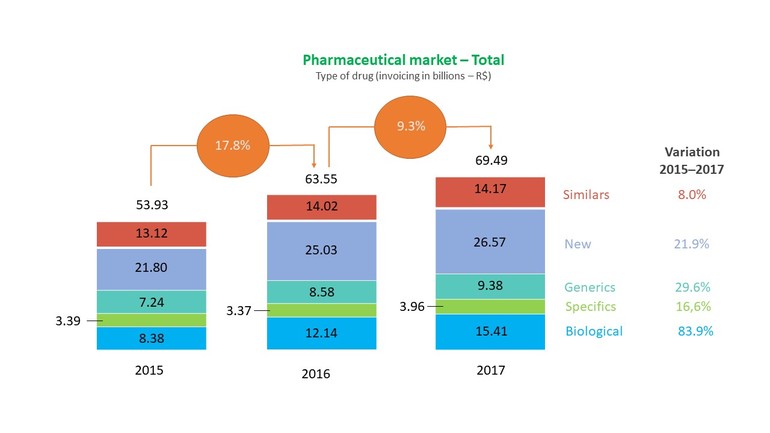
New drugs have the largest share of the pharmaceutical market, with around R$26.5 billion, while biological drugs were the ones that grew, with an increase of 84% between 2015 and 2017.
Figure 1 shows the scenario of the pharmaceutical market according to types of medicines (turnover in billions – R$). The data are taken from the report of Brazil’s National Health Surveillance Agency (Agência Nacional de Vigilância Sanitária, ANVISA) (2017 and 2018).
Figure 1: Pharmaceutical market scenario
Source: Adapted from Anvisa 2018; Anvisa 2017
The US Center for Medicare and Medicaid Services (CMS), a federal agency of the Department of Health and Human Services and a global research benchmark, projected an increase in spending with speciality drugs, including biologicals and biosimilars, of 6% per year over the period 2015–2022, and not only in value, but in use as well. The data available from 2014 is that 31% of the total cost of medicines relates to this group, although it only corresponds to 1% of the amount of prescriptions.
The following series of two articles show the market outlook from 2016 to 2025 for these medicines and a comparison of the development costs of the different types of medicines: generics, biosimilars and originator biologicals.
Related articles
Comparison of the cost of development of biologicals and biosimilars
Market outlook for biological medicines in Brazil 2016‒2025
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View this week’s headline article: Biosimilares aprobados en Colombia Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de esta semana: Biosimilares aprobados en Colombia !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
Reference
1. Tafla C. O papel dos biossimilares na saúde. [The role of biosimilars in health]. Portuguese.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2022 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment