The US Department for Health and Human Security (HSS) has selected the first drugs for Medicare price negotiation.
On 29 August 2023, HSS, through the Centers for Medicare & Medicaid Services (CMS), announced the first 10 drugs covered under Medicare Part D selected for negotiation.
This comes as a first step following the Inflation Reduction Act (IRA) which is designed to address the rapid rate of increase in Part B drug spending and lower costs for Medicare enrollees [1]. Medicare is now able to negotiate prices directly with drug companies, with the aim of lowering prices on some of the costliest prescription drugs. The negotiations with participating drug companies will occur in 2023 and 2024, and any negotiated prices will become effective beginning in 2026. HSS announced that Medicare enrollees taking the 10 drugs paid a total of US$3.4 billion in out-of-pocket costs in 2022 for these drugs.
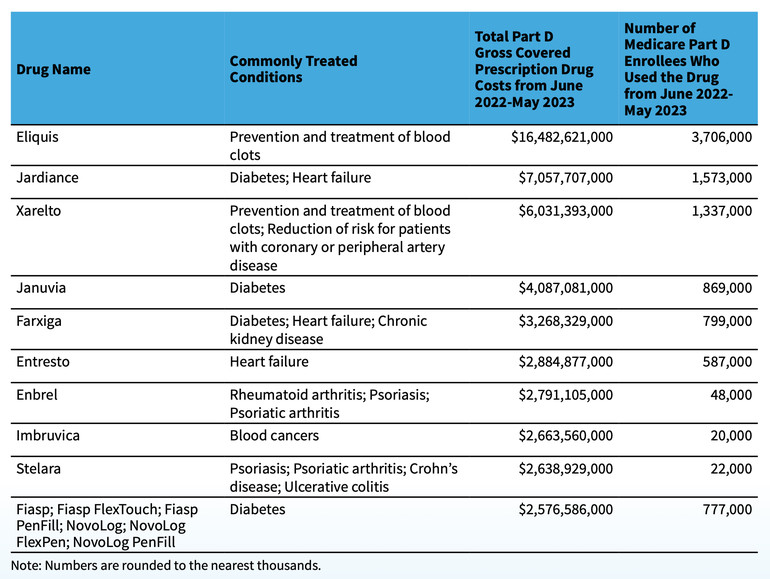
The selected drug list for the first round of negotiation is:
The negotiated price for these drugs will be announced by the CMS by September 2024. As outlined in the IRA, the next steps will be for CMS to select up to 15 more drugs covered under Part D for negotiation for 2027, up to 15 more drugs for 2028 (including drugs covered under Part B and Part D), and up to 20 more drugs for each year after that [2].
Related articles
Medicare drug price negotiation: what next?
Regulating drug prices in Medicare unlikely to lead to ‘revenue targeting’
A small number of drugs account for most of Medicare spending
|
LATIN AMERICAN FORUM
The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View the latest headline article: Biosimilares oftalmológicos en Canadá: la perspectiva de un prescriptor Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO
Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Ver el último artículo de cabecera: Biosimilares oftalmológicos en Canadá: la perspectiva de un prescriptor !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. GaBI Online - Generics and Biosimilars Initiative. Inflation Reduction Act explained [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Sep 22]. Available from: www.gabionline.net/policies-legislation/inflation-reduction-act-explained
2. GaBI Online - Generics and Biosimilars Initiative. New guidance for Medicare Drug Price Negotiation Program [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Sep 22]. Available from: www.gabionline.net/policies-legislation/new-guidance-for-medicare-drug-price-negotiation-program
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment