US Food and Drug Administration’s (FDA) advisors have voted to recommend the approval of Sandoz’s biosimilar version of Amgen/Pfizer’s arthritis blockbuster Enbrel (etanercept).
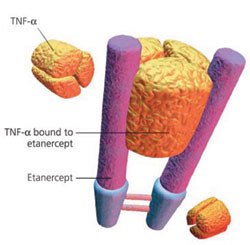
Etanercept is a biological drug that treats autoimmune diseases by inhibiting tumour necrosis factor (TNF); a soluble inflammatory cytokine. Etanercept is indicated for the treatment of rheumatoid, juvenile rheumatoid and psoriatic arthritis, plaque psoriasis and ankylosing spondylitis. Rheumatoid arthritis and psoriasis are estimated to affect around 1.3 million and 7.5 million people, respectively, in the US.
At their meeting on 13 July 2016, advisors from the FDA’s Arthritis Advisory Committee voted 20-0 in favour of the recommendation to approve Sandoz’s etanercept biosimilar (GP2015) in all five indications of the originator product (Enbrel).
The recommendation was provided after the presentation of data from a global development programme including analytical, preclinical and clinical studies of the Sandoz biosimilar etanercept, which demonstrated biosimilarity to the reference product. Clinical studies included four comparative pharmacokinetic (PK) studies in 216 healthy volunteers and a confirmatory efficacy and safety similarity study in 531 patients with chronic plaque psoriasis.
Amgen/Pfizer’s arthritis blockbuster Enbrel (etanercept) had sales of US$8.3 billion in 2014, making it one of the top selling biologicals. The patents on Enbrel will expire in the US in November 2028, after Amgen was granted a new patent, and expired in Europe in August 2015 [1, 2].
Rival biosimilars maker Samsung Bioepis has already had an etanercept biosimilar approved in Europe (Benepali) [3] and in South Korea (Brenzys) [4].
Related articles
FDA accepts application for pegfilgrastim biosimilar
Biosimilars of etanercept
References
1. GaBI Online - Generics and Biosimilars Initiative. US$67 billion worth of biosimilar patents expiring before 2020 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2016 Jul 29]. Available from: www.gabionline.net/Biosimilars/General/US-67-billion-worth-of-biosimilar-patents-expiring-before-2020
2. GaBI Online - Generics and Biosimilars Initiative. New Amgen Enbrel patent could block biosimilars until 2028 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2016 Jul 29]. Available from: www.gabionline.net/Biosimilars/News/New-Amgen-Enbrel-patent-could-block-biosimilars-until-2028
3. GaBI Online - Generics and Biosimilars Initiative. EMA recommends approval of etanercept biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2016 Jul 29]. Available from: www.gabionline.net/Biosimilars/News/EMA-recommends-approval-of-etanercept-biosimilar
4. GaBI Online - Generics and Biosimilars Initiative. Biosimilar approved in South Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2016 Jul 29]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-South-Korea
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2016 Pro Pharma Communications International. All Rights Reserved.
Source: US FDA, Novartis, Sandoz








 0
0










Post your comment