In November 2024, at the American College of Rheumatology (ACR) Convergence 2024, Alvotech shared the positive results of the primary endpoint of their comparable safety and efficacy study for their golimumab proposed biosimilar, AVT05 [1]. This is a biosimilar candidate to Janssen Biotech’s anti-inflammatory drug product, Simponi.
This comes after the April and June 2024 news of the promising topline result from the pharmacokinetic clinical study (AVT05-GL-P01, NCT05632211) for AVT05 [2].
Golimumab is a recombinant human IgG1қ monoclonal antibody (mAb) that exhibits multiple glycoforms. It blocks the binding of tumour necrosis factor-alpha (TNF-α) to its receptors, thereby reducing TNF-α-mediated inflammation. Golimumab is approved for the treatment of inflammatory conditions including rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis.
In this latest study, Alvotech assessed comparable efficacy of AVT05 and the reference product (RP) in a confirmatory efficacy and safety study (NCT05842213) in patients with moderate to severe RA. They carried out a randomized, double-blind, 2-arm, parallel group, active control study. 502 participants were randomized (1:1) and received AVT05 (n = 251) or the reference product (n = 251), 50 mg subcutaneously every 4 weeks to Week 12 inclusive. Randomization was stratified by baseline Disease Activity Score-28 for RA using C-reactive Protein (DAS28-CRP) score (≤5.1 and >5.1). The primary endpoint was change from baseline in DAS28-CRP at Week 16.
The poster presentation outlined the study’s key findings.
1. Efficacy (Week 16):
- The least squares mean difference between AVT05 and reference product was within the prespecified equivalence margin (-0.6 to 0.54), demonstrating comparative efficacy.
- Sensitivity analyses confirmed the robustness of the primary endpoint estimates.
- Subgroup analyses showed no notable differences.
2. Safety:
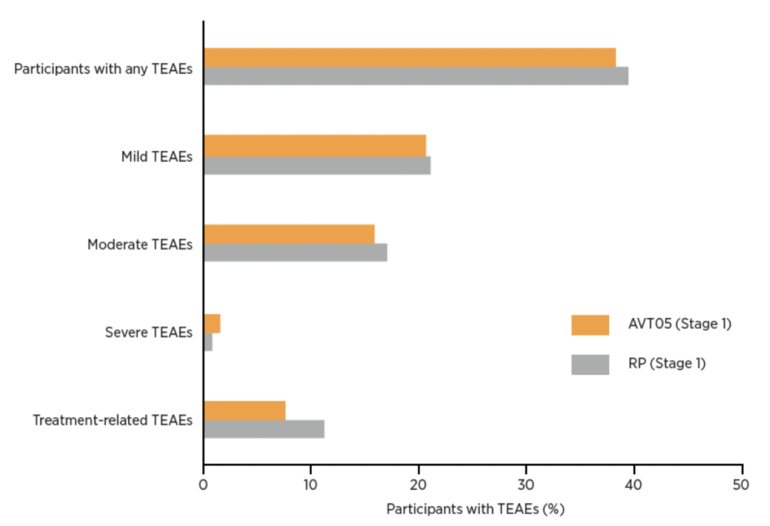
- Up to Week 16, treatment-emergent adverse events (TEAEs) were similar between groups (38.2% for AVT05 vs. 39.4% for RP), see Figure 1. Most TEAEs were mild.
- Serious TEAEs occurred in 4 AVT05 participants and 2 reference product participants, with 1 case in each group considered treatment-related, leading to study discontinuation.
Figure 1: Summary of TEAEs reported up to week 16
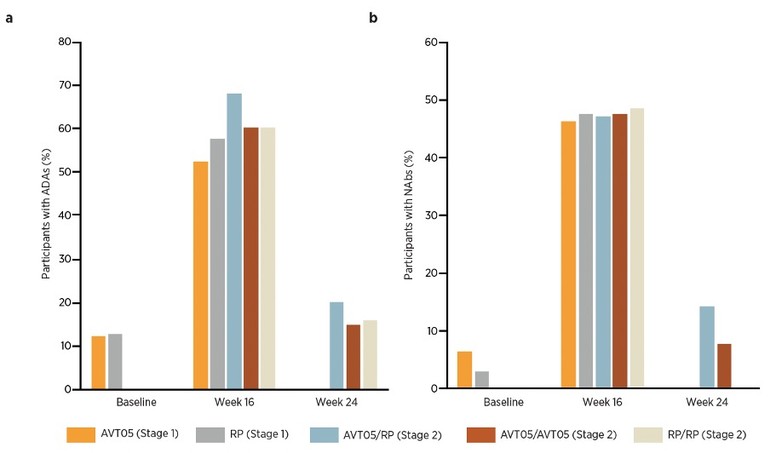
3. Immunogenicity (see Figure 2):
- At Week 16, rates of anti-drug antibodies (ADAs) were similar (52.7% in AVT05 and 57.8% in reference groups), as were rates of neutralizing antibodies (nAbs) (46.6% vs. 47.6%).
Figure 2: Positive antibody incidence in Stage 1 and Stage 2: a) ADAs; b) NAbs (safety analysis set)
4. Post-Switch Outcomes (Week 24):
- No significant differences in safety or immunogenicity were observed, including among participants who switched from reference to AVT05.
At time of presenting, the study was incomplete and will continue to Week 52 to further assess long-term outcomes.
Overall, it was concluded that that analysis of the change in DAS28-CRP from baseline to Week 16 supports the assessment of comparative efficacy between AVT05 and the reference product. In addition, the biosimilar had a safety and immunogenicity profile similar to that observed for the reference product up to Week 16, persisting to Week 24.
Alvotech was the first company to present clinical data for a proposed biosimilar to Simponi. On 4 November 2024, the European Medicines Agency accepted its marketing authorization application for AVT05. This followed the agency's earlier acceptance, on 10 October 2024, of the marketing application for AVT03, a proposed biosimilar to Prolia and Xgeva (denosumab).
Alvotech’s adalimumab (AVT02) and ustekinumab (AVT04) biosimilars were approved and/or launched in 2024, with six additional biosimilar candidates currently in development, including AVT05 [2].
Related articles
Long-term data support the clinical comparability of AVT04 to Stelara
Clinical study advances for Alvotech golimumab and Dr Reddy’s rituximab biosimilars
References
1. Assessment of Comparative Efficacy Between Candidate Biosimilar AVT05 and Reference Golimumab
https://acrabstracts.org/abstract/assessment-of-comparative-efficacy-between-candidate-biosimilar-avt05-and-reference-golimumab/
2. GaBI Online - Generics and Biosimilars Initiative. Topline results from clinical development programme for candidate biosimilar AVT05 golimumab [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2025 Jan 28]. Available from: www.gabionline.net/biosimilars/research/topline-results-from-clinical-development-programme-for-candidate-biosimilar-avt05-golimumab
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2025 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment