Sales of biologicals have almost doubled from US$63.8 billion in 2006 to US$124.6 billion in 2012.
During this time period, the best selling therapeutic classes have also changed. Back in 2006, erythropoietin biologicals were at the top of the sales league with sales of US$11.9 billion. While in 2012 erythropoietins have dropped to sixth position, with sales of US$7.2 billion, and therapeutic antibodies have taken prime position on the sales leader board. Sales of antibody biologicals accounted for 51.8% of total biologicals sales in 2012 compared to only 34.5% in 2006.
The number of blockbuster biologicals, i.e. products with sales of more than US$1 billion per year, has also increased since 2006. In 2006, there were just 20 blockbuster biologicals, whereas in 2012 this number has increased to 33.
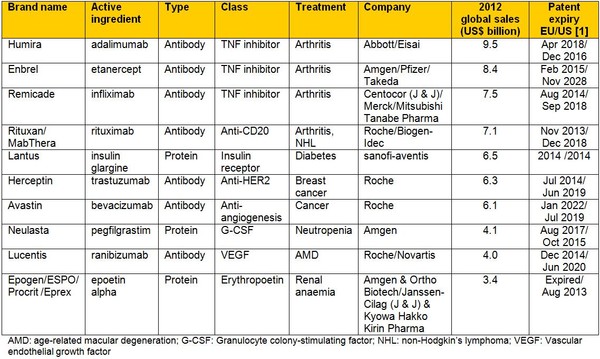
Seven of the top 10 blockbuster biologicals are antibodies, the top selling therapeutic proteins are insulin glargine and pegfilgrastim. The anti-TNF antibody Humira (adalimumab) is the top selling single brand of a recombinant biological; with 2012 sales of US$9.5 billion, see Table 1.
Table 1: Top 10 blockbuster biologicals 2012
The patents on almost all of these biological blockbusters will be expired by 2020, apart from Enbrel (etanercept) where the US patent has been extended to 2028 [2]. Considering just these 10 biologicals, this will open up around US$63 billion in sales to competition from biosimilars. The biosimilars market earned revenue of approximately US$172 million in 2010 and with global sales of biologicals on the rise, this can only increase.
Editor’s comment
If you would like to receive a high-resolution copy of Table 1*, please send us an email.
*For profit organisations subjected to a fee
Related articles
US approvals of biologicals doubled in last decade
2012’s biggest patent expiries
References
1. GaBI Online - Generics and Biosimilars Initiative. US$67 billion worth of biosimilar patents expiring before 2020 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Jun 7]. Available from: www.gabionline.net/Biosimilars/General/US-67-billion-worth-of-biosimilar-patents-expiring-before-2020
2. GaBI Online - Generics and Biosimilars Initiative. New Amgen Enbrel patent could block biosimilars until 2028 [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Jun 7]. Available from: www.gabionline.net/Biosimilars/News/New-Amgen-Enbrel-patent-could-block-biosimilars-until-2028
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment