In May 2025, South Korea’s Samsung Biologics announced that it will undergo split to separate its contract development and manufacturing organization (CDMO) business from its biosimilar and novel drug development business, Samsung Bioepis. Following this, the company announced that its CDMO arm has secured two contract manufacturing deals worth circa US$320 million with European and Asian pharmaceutical companies.
The company noted that the separation comes as part of a broader restructuring aimed at eliminating any conflicts of interest between Samsung Biologics and Samsung Bioepis. The later was set up to develop biosimilars but is increasingly involved in novel drug development. It is hoped that the split will enhance each entity’s strategic focus and reduce potential concerns over intellectual property conflicts that could arise with ongoing parallel activities in biosimilar development.
In the company announcement, Samsung Biologics said that it will establish a new holding company ‘Samsung Epis Holdings’ to oversee Samsung Bioepis and other biosimilar and new drug development businesses. Samsung Epis Holdings will be created via a spin-off scheme, with shareholders of Samsung Biologics receiving proportional stakes in both the existing CDMO business entity and the new company. Following the spin-off, Samsung Bioepis will become a wholly owned unit of Samsung Epis Holdings.
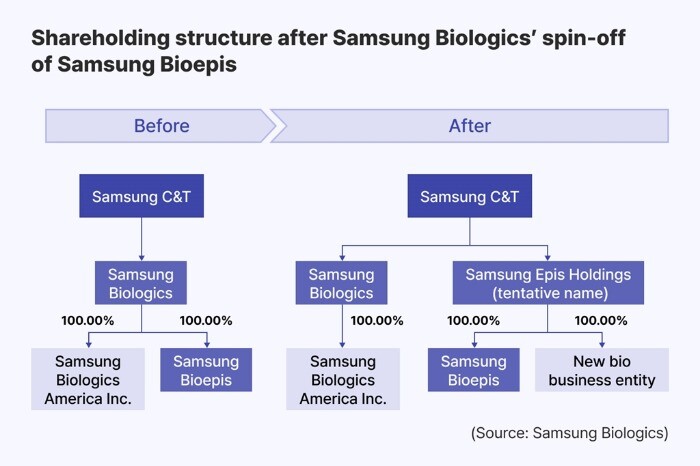
John Rim, CEO of Samsung Biologics, said: ‘The separation enables both entities to respond swiftly and decisively to the fast-evolving global biotech environment. By focusing on their respective strengths, both can secure leading positions in their fields.’
Overall, it is thought that the move will clarify the business structure, unlock corporate value and reinforce Samsung Group’s presence in the high-margin pharmaceuticals sector.
In recent years the group has focussed more and more on its biotech arm. For example, in April 2025, Samsung Biologics began full operations at its fifth plant, adding 180,000 lt of capacity facilitating a total global capacity of 784,000 lt. By 2032, the company plans to have built three more plants. This arm of the company is its strongest in terms of growth and earnings.
Related articles
Alvotech acquires Xbrane
Tocilizumab and pembrolizumab biosimilar advances for Korean firms
Ranibizumab biosimilar Ximluci and Amelivu to launch in the UK and South Korea
|
LATIN AMERICAN FORUM
The objective of GaBI’s Latin American Forum is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View the latest headline article: Alcanzar los objetivos ASG en el desarrollo farmacéutico Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO
El objetivo del Foro Latinoamericano de GaBI es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Ver el último artículo de cabecera: Alcanzar los objetivos ASG en el desarrollo farmacéutico !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2025 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment