Generics/General
|
Posted 29/06/2012
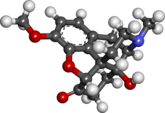
‘The old order changeth, yielding place to new’ [1]
For many years the Trade Related Intellectual Property Rights (TRIPS) negotiations have been criticised as being overly protective of the rights of large pharmaceutical companies. ‘It is unacceptable to threaten developing countries aiming to provide medicines to their populations, and disregarding international commitments to ensure access to medicines,’ ran a Médecins sans Frontières statement in 2010. ‘The US is using its trade laws to bully developing countries into applying arbitrary pharmaceutical industry requests at the expense of millions of people who depend on generic medicines in developing countries.’