Europe is way ahead of the US in terms of biosimilars regulation. A legal framework for approving biosimilars in the EU was established in 2003 and guidelines for an abbreviated registration process were issued in 2006 [1].
EMA approved its first biosimilar for somatropin (Omnitrope) back in 2006. To date, the agency has approved 14 biosimilars for use in the EU, within the product classes of human growth hormone, granulocyte colony-stimulating factor (G-CSF) and erythropoietin [2].
In Europe, guidelines are already in place covering general topics such as quality, efficacy and safety, as well as product specific issues concerning, e.g. soluble insulin, human growth hormone (somatropin), G-CSF erythropoietins, interferon-alpha, low molecular weight heparins and monoclonal antibodies [3].
In the US, however, the situation is somewhat different. Although a legal pathway was put into place with the signing into law of the Biologics Price Competition and Innovation Act of 2009 (BPCI Act) on 23 March 2010 by President Barack Obama, FDA is still in the process of developing guidelines regarding these types of products [4].
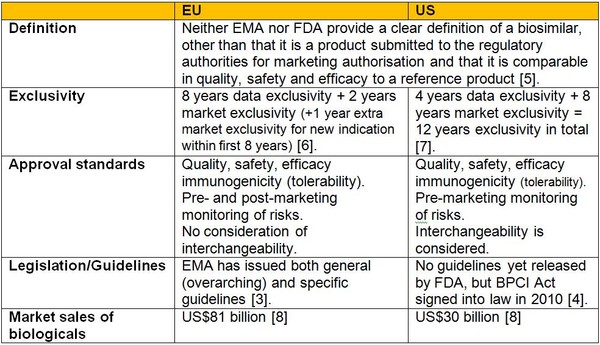
A comparison of the biosimilars pathways in Europe and the US is given in Table 1.
Table 1: Registration of biosimilars in Europe and the US
Source: Simoens S et al. [9].
Related article
Reimbursement of biosimilars
Pricing of biosimilars
Comparability studies and substitution of biosimilars
Biosimilars: demonstrating ‘similarity’
Factors affecting market access of biosimilars
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars use in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Feb 10]. Available from: www.gabionline.net/Reports/Biosimilars-use-in-Europe
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Feb 10]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
3. GaBI Online - Generics and Biosimilars Initiative. EMA and FDA to collaborate on biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Feb 10]. Available from: www.gabionline.net/Biosimilars/News/EMA-and-FDA-to-collaborate-on-biosimilars
4. GaBI Online - Generics and Biosimilars Initiative. US guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Feb 10]. Available from: www.gabionline.net/Guidelines/US-guidelines-for-biosimilars
5. GaBI Online - Generics and Biosimilars Initiative. EMA definitions of generics and biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Feb 10]. Available from: www.gabionline.net/Biosimilars/General/EMA-definitions-of-generics-and-biosimilars
6. GaBI Online - Generics and Biosimilars Initiative. Generic applications in the EU, patents and exclusivity [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Feb 10]. Available from: www.gabionline.net/Reports/Generic-applications-in-the-EU-patents-and-exclusivity
7. Title VII of the Patient Protection and Affordable Care Act of 2010, Improving Access to Innovative Medical Therapies-Subtitle (Biologics Price Competition and Innovation Act of 2009, section 351(k), 351(l), 351(m). Pub.L.No.111-48. 2011. 3-2-2011.
8. Roger SD. Biosimilars: current status and future directions. Expert Opin Biol Ther. 2010;10:1011-8.
9. Simoens S, Verbeken G, Huys I. Market access of biosimilars: not only a cost issue. Oncologie. 2011;13(5):218-21.








 0
0











Post your comment