US Food and Drug Administration (FDA) researchers are studying the use of pharmacodynamic (PD) biomarkers to demonstrate biosimilarity. These studies could make the process of developing biosimilars more efficient and faster.
Biosimilar products are highly similar to FDA-approved reference products with no meaningful clinical differences. They can bring competition and more affordable treatments. FDA and stakeholders are working on streamlining the clinical studies to facilitate efficient biosimilar development.
Presently, most biosimilar approvals require pharmacokinetic (PK) similarity data (data on how the body absorbs, distributes, metabolizes, and excretes a product) and comparative clinical studies (a study whereby investigators compare the biosimilar and reference product using clinical endpoints in a patient population) which is generally time-consuming, costly, and needs a large number of participants.
However, a more streamlined approach would be submitting PK and PD similarity data (data on the relationship between drug exposure, such as dosage levels, and the body’s subsequent response) alongside comparative safety and immunogenicity data, gathered from shorter and less expensive studies. Given that, biosimilars could be approved based on PK and PD biomarker data without a comparative clinical efficacy study [1].
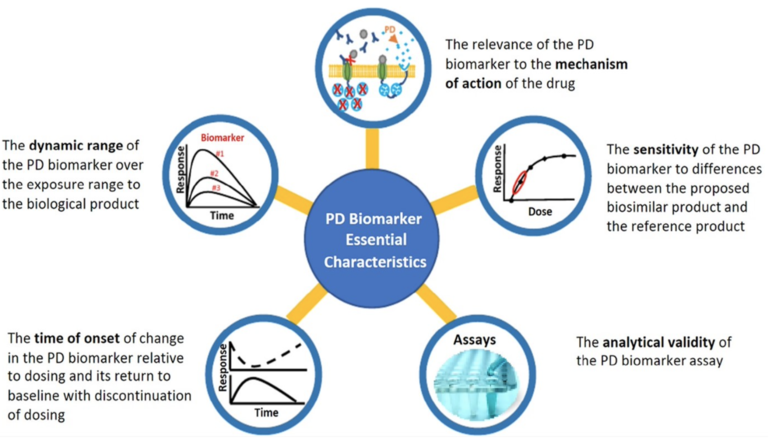
FDA has described, see Figure 1, five essential characteristics of a PD biomarker for biosimilars to help sponsors planning to use PD biomarkers in biosimilar development [2].
Figure 1: Characteristics for PD biomarkers [3]
FDA has been conducting applied research on PD biomarkers to aid in biosimilar development, as stated in the 2018 Biosimilars Action Plan. This research involves clinical pharmacology studies in which participants were administered with varying doses of a biological to participants and assessing the biomarkers' response. Biomarkers that show a relationship between dose and response may be suitable for a PD similarity study.
FDA and external researchers have identified and evaluated biomarkers using advanced technologies and simulations in placebo-controlled studies, which were published in the January 2023 issue of Clinical Pharmacology and Therapeutics.
The research has been conducted by FDA on biomarkers in the following four classes of biologicals [4]:
- PCSK9 inhibitors (Cholesterol Medications)
- IL-5 antagonists (Asthma Medications)
- Proteomics to identify IFNβ-1a biomarkers (Multiple Sclerosis Drugs)
- Model-based approach for dose selection of pegfilgrastim (Supportive Care Medications for Cancer).
The FDA's research has provided new perspectives on the analysis of PD biomarker data, enhanced our comprehension of bioanalytical considerations of analysing PK and PD endpoints, and illustrated how emerging ‘omics’ techniques can help recognize potential PD biomarkers for biosimilar development.
Related articles
The ‘positioning’ of PD biomarkers in evaluating biosimilarity
PD biomarkers for biosimilar development and approval
FDA to investigate PD biomarkers to show biosimilarity
|
LATIN AMERICAN FORUM
The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View the latest headline article: Avanzar en el desarrollo de medicamentos biosimilares con biomarcadores farmacodinámicos Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO
Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Ver el último artículo de cabecera: Avanzar en el desarrollo de medicamentos biosimilares con biomarcadores farmacodinámicos !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. GaBI Online - Generics and Biosimilars Initiative. The role of PD biomarkers in biosimilarity [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Apr 27]. Available from: www.gabionline.net/reports/the-role-of-pd-biomarkers-in-biosimilarity
2. GaBI Online - Generics and Biosimilars Initiative. Key considerations for PD biomarkers in evaluating biosimilarity [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Apr 27]. Available from:
www.gabionline.net/reports/key-considerations-for-pd-biomarkers-in-evaluating-biosimilarity
3. Strauss DG, Wang YM, Florian J, et al. Pharmacodynamic Biomarkers Evidentiary Considerations for Biosimilar Development and Approval. Clin Pharmacol Ther. 2023, 55-61. doi.org/10.1002/cpt.2761.
4. Innovations in Biosimilars. Clin Pharmacol Ther. 2023;113(1):1-195. doi:10.1002/cpt.2653
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.








 0
0











Post your comment