The US Food and Drug Administration (FDA) was busy in June 2024. On 28 June 2024, the FDA granted approval for Formycon’s Ahzantive (aflibercept-mrbb), making it the third approval for a biosimilar referencing Eylea.
The announcement of Ahzantive/FYB203 by Formycon and its licensing partner Klinge Pharma follows the news of approvals of Biocon Biologic’s Yesafili (aflibercept-jbvf) and Samsung Bioepis and Biogen's Opuviz (aflibercept-yszy) as the first interchangeable biosimilars to Regeneron and Bayer's Eylea [1].
FDA has approved biosimilar Ahzantive (aflibercept-mrbb) in the formulations of 2 mg/0.05 mL of 40 mg/mL injection.
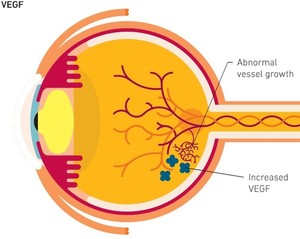
Aflibercept is a vascular endothelial growth factor (VEGF) inhibitor [2], a recombinant fusion protein consisting of VEGF-binding portions from the extracellular domains of human VEGF receptors 1 and 2, that are fused to the Fc portion of the human IgG1 immunoglobulin.
Ahzantive received FDA approval for treating patients with age-related neovascular (wet) macular degeneration (nAMD) and other serious retinal diseases, including diabetic macular oedema, diabetic retinopathy, and macular oedema following retinal vein occlusion. FDA’s decision was based on a comprehensive data package including research comparing the safety, efficacy, and tolerability of Ahzantive (FYB203) with that of Eylea in patients with nAMD.
A marketing authorization application for FYB203 was also submitted to the European Medicines Authority (EMA) at the end of 2023, with a decision anticipated by early 2025.
Related articles
Formycon launches biosimilar ranibizumab Ravegza in Saudi, Gedeon Richter invests
Biosimilars lawsuits and settlement updates for Regeneron and Alvotech
Ophthalmology biosimilars in Canada: a prescriber’s perspective
|
LATIN AMERICAN FORUM
The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View the latest headline article: Richmond lanzará los biológicos similares bevacizumab Yriviak y adalimumab Armixa Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO
Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Ver el último artículo de cabecera: Richmond lanzará los biológicos similares bevacizumab Yriviak y adalimumab Armixa !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa.
|
References
1. GaBI Online - Generics and Biosimilars Initiative. FDA approves first interchangeable aflibercept biosimilars to treat macular degeneration [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Aug 13]. Available from: www.gabionline.net/biosimilars/news/fda-approves-first-interchangeable-aflibercept-biosimilars-to-treat-macular-degeneration
2. GaBI Online - Generics and Biosimilars Initiative. Biosimilars of aflibercept [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2024 Aug 13]. Available from: www.gabionline.net/biosimilars/general/biosimilars-of-aflibercept/
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2024 Pro Pharma Communications International. All Rights Reserved.








 0
0










Post your comment