Biosimilars
WHO revised guidelines for biosimilars: scientific background
A study carried out by Kurki P et al. in 2022 reviewed the current clinical experience and scientific evidence to provide an expert perspective for updating the World Health Organization (WHO) guidelines on evaluation of similar biotherapeutic products (SBPs; also called biosimilars) to increase flexibility and clarity.
EMA accepts application for high concentration adalimumab biosimilar
Sandoz, the generics division of Novartis, announced on 17 June 2022 that the European Medicines Agency (EMA) has accepted the application for its high concentration formulation (HCF) of its adalimumab biosimilar Hyrimoz (GP2017).
Positive phase III results for Samsung Bioepis’ Soliris biosimilar
Samsung Bioepis announced that its biosimilar to Soliris (eculizumab) has comparable efficacy and safety and is bioequivalent to the originator.
EMA recommends approval of bevacizumab biosimilar Vegzelma
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) announced on 24 June 2022 that it had recommended granting marketing authorization for the bevacizumab biosimilar Vegzelma.
Adalimumab biosimilar MSB11022: PK and tolerability of autoinjector versus pre-filled syringe
The antitumour necrosis factor-alpha (anti-TNF‑a) monoclonal antibody adalimumab is used to treat a variety of chronic immune-mediated inflammatory diseases, including rheumatoid arthritis, psoriasis, psoriatic arthritis, juvenile arthritis and inflammatory bowel disease. The biosimilar MSB11022 (Idacio/Kromeya) has demonstrated physicochemical and functional similarity to reference adalimumab (Humira) in the preclinical setting. Further study in the clinical setting has established equivalent pharmacokinetics (PK) and efficacy, and comparable safety and immunogenicity for MSB11022 versus adalimumab. MSB11022 is available in three delivery formats: pre-filled syringe, vial and autoinjector. Offering a choice of devices can address patient needs and potentially improve adherence to therapy. Self-injection via pre-filled syringes may be challenging for some patients, due to issues such as needle phobia, pain-related concerns and arthritis hand pain making self-injection more difficult. The availability of alternative self-injection devices allows patients to select a device that suits their needs.
American Academy of Ophthalmology biosimilars guidance details
The American Academy of Ophthalmology (AAO) recognizes the potential societal value of biosimilars for improving care of patients with eye disease. Biosimilars should have sufficient research demonstrating their safety and effectiveness for treatment of eye disease. In January 2022, the AAO issued a policy statement on the biosimilars in ophthalmologic use [1].
No trends in biosimilars uptake levels in the US, reveals study
There are no consistent trends in biosimilar uptake by order of market entry, care setting, or pricing, finds a new study published in the Journal of General Internal Medicine [1]. In addition, biosimilar uptake is generally lower than that reported for generic drugs in the US.
FDA approves pegfilgrastim and rituximab biosimilars
The US Food and Drug Administration (FDA) has approved the pegfilgrastim biosimilar Fylnetra (pegfilgrastim-pbbk) and the rituximab biosimilar Riabni (rituximab-arrx).
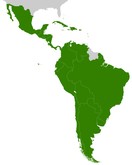
Current status of monoclonal antibody biosimilars approved in Latin America
By the end of 2021, biosimilar antibodies of rituximab, trastuzumab, infliximab, adalimumab and bevacizumab were expected to be commercialized in Latin America with 25 different brand names.
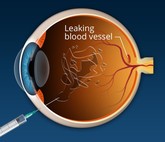
Byooviz: first ophthalmology biosimilar launches in US
Biogen and Samsung Bioepis have launched Byooviz (ranibizumab-nuna) on the US market as the first ophthalmology biosimilar. It references Roche’s (Genentech’s) blockbuster therapy, Lucentis.