Biosimilars
FDA approves third interchangeable ranibizumab biosimilar Nufymco
On 18 December 2025, the US Food and Drug Administration (FDA) granted approval to Nufymco (ranibizumab-leyk), an interchangeable biosimilar to Genentech’s Lucentis (ranibizumab).
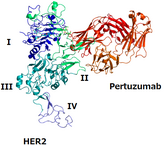
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
On 13 November 2025, the US Food and Drug Administration (FDA) approved Shanghai Henlius Biologics’ Poherdy (pertuzumab-dpzb) as an interchangeable biosimilar to Genentech’s Perjeta. Additionally, on 28 November 2025, the FDA approved Lupin’s pegfilgrastim biosimilar, Armlupeg (pegfilgrastim-unne).
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
On 13 November 2025, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted positive opinions positive opinions recommending the granting of a marketing authorization for two biosimilar medicines: Ondibta (insulin glargine), for the treatment of diabetes mellitus; and Osqay (denosumab), for the treatment of osteoporosis and bone loss.
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
The US Food and Drug Administration (FDA) approved four denosumab biosimilars in late 2025. The first two, Osvyrti and Jubereq (denosumab-desu), were approved on 29 October 2025, and reference Amgen’s Prolia and Xgeva, respectively. The second pair, Boncresa and Oziltus (denosumab-mobz), were approved on 19 December 2025, and also reference Prolia and Xgeva.
FDA approves aflibercept biosimilar Eydenzelt and label expansion for adalimumab biosimilar Yuflyma
On 9 October 2025, the US Food and Drug Administration (FDA) approved Celltrion’s Eydenzelt (aflibercept-boav), a biosimilar referencing Regeneron’s Eylea (aflibercept). On 17 October 2025, the FDA approved expanded indications for Celltrion’s Yuflyma (adalimumab-aaty) and its unbranded version.
ANVISA approves biosimilars for denosumab, trastuzumab, and aflibercept
In September 2025, ANVISA, the Brazilian Health Regulatory Agency, issued favourable opinions recommending marketing authorization for four biosimilars, including two biosimilars of denosumab, one of trastuzumab, and one of aflibercept [1].
Biosimilars referencing Amgen’s Neulasta and Neupogen launch in Canada and US
Kashiv BioSciences has launched two biosimilars, Pexegra and Filra, in Canada, while Tanvex Biopharma has introduced its biosimilar, Nypozi (filgrastim-txid), to the US market, expanding patient access in North America.
EMA recommends approval for nine biosimilars
On 18 September 2025, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted positive opinions recommending the granting of a marketing authorization for nine biosimilars, including seven denosumab, one golimumab, and one ustekinumab biosimilar medicines.
FDA approves six denosumab biosimilars
In August and September 2025, the US Food and Drug Administration (FDA) approved six denosumab biosimilars, expanding treatment options for patients with bone health issues.
EMA recommends approval for four biosimilars targeting three therapies
On 24 July 2025, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) adopted positive opinions recommending the granting of a marketing authorization for four biosimilars, including two denosumab biosimilar medicines, an aflibercept biosimilar, and an ustekinumab biosimilar.