Biosimilars
Recommendations for improving biosimilar regulations in Latin America
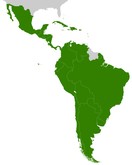
The biosimilar regulatory scenario is very diverse and varies widely in the large group of Latin American countries. Authors Teran et al. presented some recommendations for making biosimilar regulations in Latin America more homogeneous and comprehensive [1].
Successful trials for Sandoz and Lannett biosimilars
On 19 September 2022, Sandoz announced positive results following its ROSALIA I/III clinical trial study for its proposed biosimilar denosumab. This follows the August announcement that Lannett Company successfully completed subject dosing in the clinical trial of its biosimilar insulin glargine.
EMA calls for biosimilar interchangeability across the EU
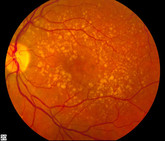
The European Medicines Agency (EMA) and the Heads of Medicines Agencies (HMA) issued on 19 September 2022 a joint statement confirming that biosimilar medicines approved in the European Union (EU) are interchangeable with their reference medicine or with an equivalent biosimilar. This will allow more patients to have access to biological medicines necessary for treating diseases such as cancer, diabetes and rheumatic diseases.
Recommendations to address challenges to biosimilars in Latin America
After reviewing the regulatory landscape for biosimilars [1] and access to biosimilars for cancer treatments in Latin America [2], authors Teran et al. advised several recommendations to address challenges related to poor access to biosimilars in Latin America healthcare systems.
EC approves bevacizumab biosimilar Vegzelma
On 18 August 2022, South Korea-based biologicals specialist Celltrion announced that its bevacizumab biosimilar, Vegzelma (CT-P16), had received European Commission (EC) approval.
EC approves ranibizumab biosimilar Ranivisio
On 29 August 2022, the ranibizumab biosimilar Ranivisio (FYB201), developed by Bioeq – a joint venture between Formycon and Polpharma Biologics, was granted marketing approval by the European Commission (EC).
FDA accepts application for high concentration adalimumab biosimilar
Sandoz, the generics division of Novartis, announced on 21 July 2022 that the US Food and Drug Administration (FDA) had accepted the supplemental biologics license application for the high concentration formulation (HCF) of its adalimumab biosimilar Hyrimoz (GP2017) (adalimumab-adaz).
Applications for natalizumab biosimilars accepted by FDA and EMA
Applications for natalizumab biosimilars made by Sandoz (the generics division of Novartis) and Polpharma Biologics (Polpharma) have been accepted by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA).
Regulatory landscape for biosimilars in Latin America
The biosimilar regulatory situation in Latin America varies broadly among the different countries, even though Latin America is moving towards consolidating defined and standardised regulatory pathways for these products. This article gives a summary of the biosimilar regulatory status for the countries represented by members of the panel of experts of the American Health Foundation (AHF). The countries reviewed are Chile, Colombia, Ecuador, Guatemala and Peru [1].
Totality of evidence for biosimilar pegfilgrastim Ziextenzo
Agarwala et al. have recently published a review on the totality of evidence (ToE) for the biosimilar pegfilgrastim Ziextenzo® (LA-EP2006) matching the European Union- (EU) and US-reference biological pegfilgrastim Neulasta® (marketed by Amgen) [1].