Biosimilars
FDA accepts application for tocilizumab biosimilar
Fresenius Kabi, the generics unit of German healthcare giant Fresenius, announced on 1 August 2022 that the US Food and Drug Administration (FDA) had accepted the application for their proposed tocilizumab biosimilar (MSB11456).
What does the designation of interchangeability for biosimilars in the US mean?
Biologicals have significantly improved patients’ quality of life, yet, access to these critical medicines are constrained due to cost and this is being exacerbated by the misperceptions around biosimilars. In particular, imprecise terminology has been applied to biosimilars, leading to the implication that biologicals designated as interchangeable by the US Food and Drug Administration (FDA) are better biosimilars. The US is the only jurisdiction in the world with this additional designation from the regulator as an option for biosimilar sponsors to consider [1].
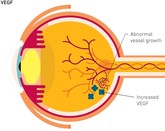
FDA approves first interchangeable ranibizumab biosimilar
Polpharma Biologics, Formycon and Bioeq jointly announced on 2 August 2022 that they had received approval from the US Food and Drug Administration (FDA) for their ranibizumab biosimilar (CHS-201/FYB201).
Access to biosimilars for cancer treatments in Latin America
In many Latin America countries, patient access to biosimilars for cancer treatment remains restricted. In particular for patients with breast cancer and colorectal cancer, biosimilars can be a step further to increasing access to care [1].
Innovent-Etana bevacizumab biosimilar approved in Indonesia
Innovent announced the approval of its bevacizumab biosimilar in Indonesia, that was developed in partnership with PT Etana Biotechnologies Indonesia.
Ranibizumab biosimilar, FYB201, receives EMA recommendation
Formycon’s ranibizumab biosimilar, FYB201, has received a positive opinion from the European Medicines Agency (EMA) Committee for Medicinal Products for Human Use (CHMP).
Biosimilar regulations perspective in Latin America to improve cancer treatment access
To address the issue of regulatory process of biosimilars, including intended copies*, the Americas Health Foundation (AHF) conducted a literature review and convened a panel with eight experts in biological cancer therapies and health economics to address the most salient issues concerning the regulation of biosimilars in Latin America [1].
Pegfilgrastim biosimilar Udenyca demonstrated similar immunogenicity to Neulasta
The pegfilgrastim biosimilar Udenyca (pegfilgrastim-cbqv) is a pegylated, long-acting form of filgrastim (granulocyte colony-stimulating factor [G-CSF]). It is approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as a biosimilar of the originator pegfilgrastim (Neulasta) for febrile neutropenia in patients receiving myelosuppressive chemotherapy [1, 2].
Therapeutic drug monitoring with infliximab improves disease control
The latest study from the Norwegian Drug Monitoring (NORDRUM) trial [1] shows that proactive therapeutic drug monitoring (TDM) for patients receiving maintenance infliximab therapy improves disease control, compared to treatment without TDM.
New data on infliximab and adalimumab biosimilars at EULAR 2022
The Annual European Congress of Rheumatology (EULAR 2022) was held at the beginning of June 2022. This platform was used to announce the results of a study that showed that, in patients with rheumatoid arthritis, statistically greater improvements in clinical outcomes with subcutaneous infliximab, compared to intravenous infliximab. In addition, Samsung Bioepis announced new data on their established adalimumab biosimilar Imraldi (Hadlima outside Europe).