Biosimilars
Evolution of biosimilars in developed and developing countries
The development of biologicals has experienced continuous growth over the past three decades. The expiration of patent protection for many biologicals has led to the development of biosimilars in many countries around the world. This paper reviews the literature on biosimilars and covers their therapeutic status, clinical trials, approved biosimilars and regulatory guidelines in Japan, South Korea and Malaysia [1].
Biosimilars: the benefits need to be communicated
Extrapolation may be the most contentious issue of biosimilar development, but it is also its single greatest benefit, says Dr Martina Weise of the Federal Institute for Drugs and Medical Devices in Germany.
Samsung Bioepis adalimumab biosimilar submitted to EMA
South Korean electronics giant Samsung and biotechnology company Biogen Idec’s joint venture Samsung Bioepis announced on 18 July 2016 that its adalimumab biosimilar candidate, SB5, had been accepted for review by the European Medicines Agency (EMA).
Sandoz plans to launch five more biosimilars by 2020
Sandoz, the generics division of Novartis, has announced plans for five major global biosimilar launches by 2020.
Biosimilars: clinicians and regulators need to talk
In Europe, there is a clear gap between the regulatory decisions that govern biosimilar approval and the recommendations of medical societies. The fact that the views of medical societies, whose members are the physicians that will prescribe biosimilars, disagree with those of regulators, may hold back biosimilar uptake.
FDA rejects Sandoz’s biosimilar pegfilgrastim application
Novartis disclosed on 19 July 2016 that the application by its Sandoz unit to market a biosimilar version of pegfilgrastim has been rejected by the US Food and Drug Administration (FDA).
Pure red cell aplasia in a CKD patient after treatment with epoetin zeta
Authors from the Versilia and Manzoni Hospitals in Italy report the case of a patient who developed pure red cell aplasia (PRCA) following subcutaneous administration of epoetin zeta, which is one of the two biosimilars of epoetin alfa licensed in Europe [1].
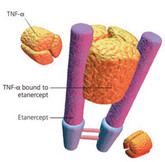
FDA advisors recommend approval of Sandoz’s etanercept biosimilar
US Food and Drug Administration’s (FDA) advisors have voted to recommend the approval of Sandoz’s biosimilar version of Amgen/Pfizer’s arthritis blockbuster Enbrel (etanercept).
Biosimilars of tocilizumab
Last update: 4 December 2020
Tocilizumab is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). It is an immunosuppressive drug used mainly for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. In Japan, tocilizumab is also approved for the treatment of Castleman’s disease, a rare benign tumour of B cells.
The economic impact of biosimilars in the US
Biologicals are large molecule compounds used to treat rare or complex diseases. Between 2013 and 2014, spending on specialty drugs, including biologicals, increased 32.4%, while spending on small-molecule drugs increased by just 6.8%. By 2016, eight of the 10 top-selling drugs are expected to be biologicals.