Biosimilars
Effectiveness of ESAs in treating anaemia in kidney disease and cancer patients
Erythropoiesis-stimulating agents (ESAs) are biological analogues of human erythropoietin used for the treatment of anaemia associated with chronic kidney disease (CKD) and chemotherapy treatment in cancer patients [1]. ESA biosimilars have been available on the Italian market since 2007. However, only limited post-marketing data exist on the comparative effectiveness of biosimilar and originator ESAs in routine care.
Biosimilars of eculizumab
Last update: 20 October 2017
Eculizumab is a humanized monoclonal antibody that is a terminal complement inhibitor. It is used to treat people with paroxysmal nocturnal haemoglobinuria (PNH), for whom it improves quality of life but does not appear to affect the risk of death. It is also indicated for the treatment of patients with atypical haemolytic uremic syndrome (aHUS) – a disease that primarily affects kidney function – to inhibit complement-mediated thrombotic microangiopathy.
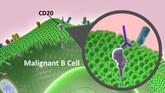
Rituximab biosimilar from Sandoz accepted for review by EMA
Sandoz, the generics division of Novartis, announced on 24 May 2016 that its regulatory submission for its proposed rituximab biosimilar (GP2013) had been accepted by the European Medicines Agency (EMA).
Samsung Bioepis infliximab biosimilar accepted for review by FDA
Samsung Bioepis (a Biogen and Samsung joint venture) and Merck announced on 24 May 2016 that the US Food and Drug Administration (FDA) had accepted for review a Biologics License Application (BLA) submitted by Samsung Bioepis for the companies’ infliximab biosimilar candidate, SB2.
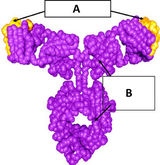
Monoclonal antibodies and the challenge of substitution
Healthcare payers are eagerly awaiting the arrival of biosimilar competition in the innovative monoclonal antibody sector in order to drive down drug prices and increase patients’ access to these medicines. As the first to introduce scientific and regulatory requirements for the approval of biosimilars in 2004, the European Union (EU) has emerged as a testing ground for biosimilars. In view of the lack of stance of EU governments and national institutions on substitution for biosimilars, hospitals and healthcare structures logically took up this major issue.
Roche sues India’s drug regulator over Avastin ‘similar biologics’
Switzerland-based drug giant Roche has sued the Drug Controller General of India (DCGI) in the Delhi High Court over ‘similar biologic’ versions of its cancer blockbuster Avastin (bevacizumab).
Pharmacokinetic behaviour of a trastuzumab biocomparables
Biosimilars represent a viable alternative for the treatment of chronic and degenerative diseases of many patients worldwide who cannot afford the costs of biotherapies based on originator products. Trastuzumab is a humanized monoclonal antibody, which is used for the treatment of HER2-positive breast cancer. In the review paper of Miranda-Hernández et al. [1], the authors described the development of a trastuzumab biocomparable by Mexico-based Probiomed. This biocomparable, according to the authors, was developed in compliance with international guidelines and the characterization of Critical Quality Attributes (CQAs), as well as the pharmacokinetic parameters evaluated in healthy volunteers, demonstrated comparability with the reference product.
Switching between different ESAs
Switching between reference biologicals and biosimilars can be a contentious issue. A study from Italy, however, has found that this phenomenon is not limited to reference products and their biosimilars, but also often occurs between originator biologicals and other originator biologicals within the same category [1].
Positive phase III results for omalizumab copy biological
US-based biopharmaceutical company Sorrento Therapeutics (Sorrento) announced on 16 May 2016 that its partner, MabTech had successfully completed a combined phase II and III clinical trial in China for STI-004, a copy biological for omalizumab (Xolair). STI 004 met its primary endpoint in a multicentre, randomized, double-blind, placebo-controlled, clinical study.
Uptake of biosimilars increasing in Spain
Use of biosimilars in Madrid, Spain, has increased after approaches were introduced to try and improve uptake of biosimilars in the country, according to Ainhoa Aranguren Oyarzábal and colleagues from the Madrid Health Service (MHS), Spain [1].