Biosimilars
Clinical pharmacists have a critical role in increasing biosimilar uptake
The increasing global burden of chronic diseases, including cancers, diabetes, autoimmune disorders, anaemia of chronic renal failure, rheumatoid arthritis, multiple sclerosis, blood disorders and others, underscore the importance of patients’ access to safe and effective treatments. Interestingly, the introduction of biologicals in the 1980s revolutionized the treatment of these chronic diseases with better prognosis, although high costs and limited patient access remain challenges. These biologicals are known by various names, including biopharmaceutical agents, biologicals, biological therapies, biological agents and biological response modifier therapy or immunotherapy. Biologicals are derived or manufactured from a living biological system. With the majority of originator biologicals losing patent protection and the emergence of biosimilars, the landscape of biologicals is facing many changes.
Clinical development of biosimilars in the oncology setting
Biologicals as monoclonal antibodies (mAb) are highly complex products produced in living systems. They are included as treatment, combined with chemotherapy, for multiple common malignancies as cancer, in first- and second-line treatment regimens. However, the patient’s access to this type of treatment can be limited due to their high cost.
Knowledge and perceptions of naming for biosimilars in the US
The relatively recent introduction of biosimilars to the US market and the new naming convention for biopharmaceuticals prompted exploration of their impact in clinical practice. Naming guidance for new biological products and biosimilars was published by the US Food and Drug Administration (FDA) in 2017, which proposed the use of a core name followed by a 4-letter suffix devoid of meaning to facilitate pharmacovigilance [1]. In order to find out how this system was viewed by healthcare providers, researchers evaluated use of, knowledge about, perceptions of, and preferences for this naming convention in clinical practice [2]. Previous studies informed the hypothesis that healthcare providers would demonstrate knowledge gaps surrounding biosimilars and opinions regarding 4-letter suffix use would be inconsistent. This study aimed to understand the impact of the recent naming guidance in clinical practice.
Mabpharm gains approval for infliximab biobetter in China
US-based biopharmaceutical company Sorrento Therapeutics (Sorrento) announced on 20 July 2021 that its partner, China-based Mabpharm, had received marketing approval from China’s National Medical Products Administration (NMPA), formerly the China Food and Drug Administration (CFDA), for its infliximab ‘biobetter’ (CMAB008).
Glosario de términos principales
Last update: 23 September 2022
Desde el lanzamiento del primer biosimilar en Europa en abril de 2006, se ha debatido de manera reiterada sobre el uso adecuado de la terminología relativa a los medicamentos biológicos, principalmente en inglés.
Interactive map for interchangeable biosimilars
US-based healthcare services company Cardinal Health has launched an interactive map for interchangeable biosimilars as part of its information for biosimilars.
New quality-range-setting method for biosimilarity assessment
Spanish researchers present a new method for the estimation of quality range (QR) bounds based on the variance components to account for both between-lots and within-lots variability; variance components are computed by the maximum likelihood method using a linear random model [1]. The authors have called this method QRML to differentiate it from the currently used procedure based on one sample per batch. For this, the molecular weight (Mw) and dimer content (expressed as percentage) were used as critical quality attributes (CQAs). Real data from seven batches of a commercial bevacizumab drug product were used.
Biosimilars and interchangeability in oncology
Researchers from Brazil investigated, from a pharmaceutical perspective, a major problem of the present time, which is the interchangeability of biosimilars [1].
Biosimilars approved in Costa Rica
In Costa Rica, the regulatory body responsible for the approval of biologicals is the Ministry of Health.
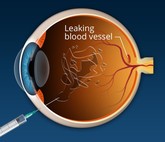
Formycon/Bioeq submit European marketing authorization for ranibizumab biosimilar
German-headquartered companies Formycon and Bioeq have announced a European marketing authorization application for their ranibizumab biosimilar, FYB201.