Biosimilars
China approves tocilizumab copy biological BAT1806
In January 2023, Bio-Thera Solutions announced that the China National Medical Products Administration (NMPA) approved BAT1806, a biosimilar of Actemra (tocilizumab), in China. This is the world’s first tocilizumab copy biological/biosimilar to be approved.
A global overview of manufacturers of follow-on biologicals
Recently, the World Health Organization (WHO) has pointed out that the absence of appropriate regulatory frameworks for biosimilars may have led to the approval of follow-on biologicals that cannot be considered biosimilars according to current WHO biosimilar guidelines, which were coined ‘non-innovator biologicals’ by WHO. In order to investigate the existence of ‘non-innovator biologicals’ in global markets, more understanding of the structure of the market and the manufacturers that are active in this field is needed.
FDA accepts application for denosumab biosimilar GP2411
Sandoz, the generics division of Novartis, announced on 6 February 2023 that the US Food and Drug Administration (FDA) has accepted its Biologics License Application (BLA) for a proposed denosumab biosimilar (GP2411).
EMA accepts application for ustekinumab biosimilar AVT04
Alvotech announced on 9 February 2023 that the European Medicines Agency (EMA) has accepted a Marketing Authorization Application for AVT04, their proposed biosimilar to Stelara (ustekinumab).
Study supports increased development of insulin biosimilars
Biosimilar insulins appear to be satisfactory in the treatment of both type 1 and type 2 diabetes and there is a strong case for increasing biosimilar insulin development, finds an editorial published in the Journal of Diabetes [1].
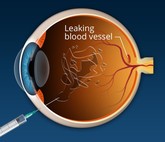
Ranibizumab biosimilar Ximluci and Amelivu to launch in the UK and South Korea
STADA and Xbrane Biopharma have announced that the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has granted a marketing authorization for Ximluci, a biosimilar referencing ophthalmology drug, Lucentis (ranibizumab). In addition, Samsung Bioepis and Samil Pharmaceuticals will launch their biosimilar of Lucentis, Amelivu, in South Korea.
Malaysian hospital pharmacists’ perspective on and role in promoting biosimilars use
This survey study carried out by Mohd Sani N et al. aimed to evaluate Malaysian pharmacists’ perspectives of biosimilars and to determine factors associated with pharmacists successfully promoting their use.
Advances for adalimumab biosimilars in Saudi Arabia, Europe and Canada
In January 2023, Alvotech-Bioventure, Sandoz and Samsung Bioepis announced advances for their adalimumab biosimilars in Saudi Arabia, Europe and Canada, respectively.
Survey demonstrates US pharmacist biosimilar knowledge gaps
Previous studies of stakeholder perceptions have primarily focused on physicians. However, pharmacists are key stakeholders in the use and adoption of biosimilars. The study carried out by Stevenson et al. sought to gain insights from US pharmacists about their knowledge and approach to the use of biosimilars.
DARS: advancing biosimilar development in the US
This article reviews the recent engagement of the Division of Applied Regulatory Science (DARS) in several current initiatives on utilizing pharmacodynamic (PD) biomarkers to demonstrate biosimilarity, potentially streamlining or negate the need for comparative clinical studies.