Biosimilar insulins appear to be satisfactory in the treatment of both type 1 and type 2 diabetes and there is a strong case for increasing biosimilar insulin development, finds an editorial published in the Journal of Diabetes [1].
Study supports increased development of insulin biosimilars
Biosimilars/Research
|
Posted 03/03/2023
 1
Post your comment
1
Post your comment
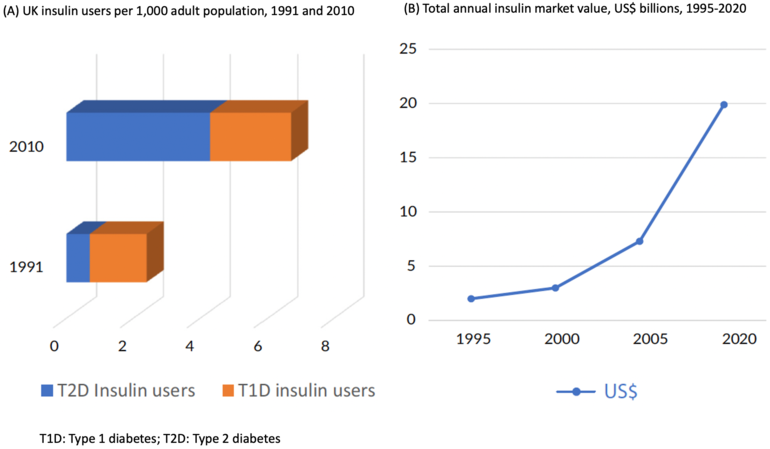
The editorial by Zachary T Bloomgarden at the Icahn School of Medicine, Mount Sinai, USA highlights that, since the early 1990s the use of insulin to treat diabetes has increased substantially, see Figure 1.
Figure 1: UK insulin users and total annual insulin market value
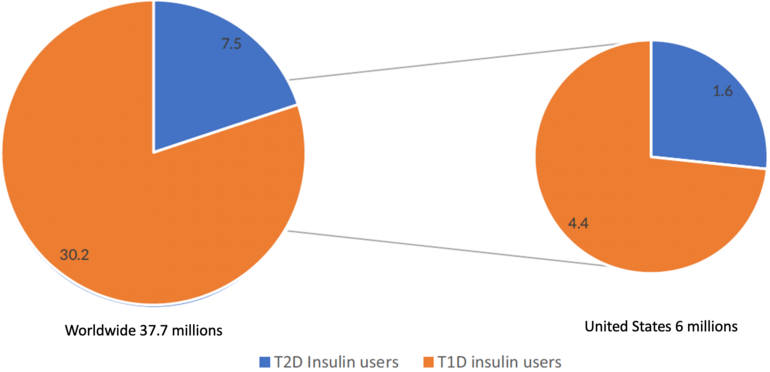
There are now an estimated 37.7 million insulin-treated diabetes patients worldwide, see Figure 2.
Figure 2: Estimated current number of insulin users with T1D and with T2D, worldwide and the US
The first long-acting insulin analogue, insulin glargine, was approved in 2020, and Bloomgarden notes that there is evidence to suggest that treatment with analogues is preferable to biosynthetic human insulin (BHI) treatment. However, treatment with insulin analogues is expensive at almost US$6,000 per insulin-treated person annually in the US. He notes that it is suggested that the use and uptake of biosimilar insulins could reduce expenditures by 50%.
Bloomgarden also highlights that the US Food and Drug Administration (FDA) distinguishes between follow-on biologicals, that are ‘highly similar to the reference product notwithstanding minor differences in clinically inactive components,’ and having ‘no clinically meaningful differences from reference product in safety, purity, and potency’; and interchangeable biosimilars, that can be substituted for one another and the reference at the pharmacy level. He gives the details of various studies to demonstrate that a given dose of insulin has highly variable expected action, despite this, a variety of biosimilar insulin preparations show satisfactory results in diabetes treatment.
Furthermore, the dosing device can play an important role in treatment and changing device can cause errors in dosing that cause more problems than changing medication.
In conclusion, there is ‘strong rationale for encouraging biosimilar insulin development and effectiveness of biosimilar insulin analogue preparations developed to date appears satisfactory. The concept of stressing interchangeability as a criterion may, however, not be as important clinically as has been thought by regulatory authorities, given the marked variation in day-to-day insulin action in persons treated with the products currently in use, and issues with different devices of supposedly interchangeable insulin preparations are quite likely to cause clinical issues’.
As long as attention is paid to potential safety issues with the products, biosimilar insulins can offer more affordable and effective treatment of diabetes, according to Bloomgarden.
Related articles
The US Biologics Competition Act and Biosimilar Red Tape Elimination Act
New and upcoming biosimilars launches in the US
US prescription drug expenditure projection report
United States legislation to improve access to insulin
|
LATIN AMERICAN FORUM View the latest headline article: Directrices revisadas de la OMS para productos biosimilares seguros y eficaces Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Directrices revisadas de la OMS para productos biosimilares seguros y eficaces !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. Bloomgarden ZT. Biosimilar insulin concepts. J Diabetes. 2022;14(4):231-5.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
Posted 01/06/2024 by keerthi
Biosimilars
This article makes a strong case for the development of biosimilar insulins to make diabetes treatment more affordable. It's great to see that biosimilars can offer effective results comparable to insulin analogues but at a lower cost. Encouraging more biosimilar development could significantly reduce healthcare expenses. Ensuring proper use of dosing devices and addressing any safety concerns will be crucial for their success.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment