Biosimilars
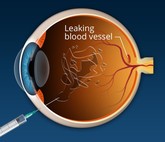
Biosimilar anti-VEGF: transforming retina treatment economics in South Asia
Anti-vascular endothelial growth factors (anti-VEGF) agents combat retinal diseases causing blindness. However, high costs and non-adherence pose challenges. Ophthalmic anti-VEGF ranibizumab biosimilars, which have received multiple approvals in South Asia, are helping to reduce healthcare costs and revolutionize ophthalmic treatments.
Long-term data support the clinical comparability of AVT04 to Stelara
Long-term efficacy, safety and tolerability data for the AVT04 ustekinumab biosimilar candidate developed in partnership by Alvotech and STADA corroborate the finding of therapeutic equivalence to the Stelara® reference product in patients with moderate-to-severe chronic plaque psoriasis (PsO) in the primary endpoint at Week 12.
FDA approves first tocilizumab biosimilar Tofidence
On 29 September 2023, the US Food and Drug Administration approved Tofidence (tocilizumab-bavi) as the first biosimilar to Actemra (tocilizumab). Tofidence is first-of-its-kind biosimilar tocilizumab to receive approval in the US.
Promotion of biosimilar product development in Puerto Rico
The Puerto Rico Economic Development Bank (Banco de Desarrollo Económico para Puerto Rico, BDE)announced in August 2023 a funding of US$3.85 million for Biosimilar Sciences PR, LLC (Biosimilar Sciences) and Ocyon Bio PR Inc (OcyonBio) to facilitate the purchase of specialized biotechnology equipment. This funding comes from the State Small Business Credit Initiative (SSBCI) of the US Treasury for Small Businesses.
FDA grants interchangeable designation to adalimumab biosimilar Abrilada
US-based pharma giant Pfizer announced on 5 October 2023 that the US Food and Drug Administration (FDA) has designated Abrilada (adalimumab-afzb) as an interchangeable biosimilar to Humira (adalimumab). The interchangeable designation applies to all approved indications of Abrilada, including certain patients with rheumatoid arthritis (RA), juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa and uveitis.
Topline results for Hadlima biosimilar in interchangeability study
Samsung Bioepis and Organon announced topline results from interchangeability study of their Humira biosimilar, SB5/Hadlima, in August 2023.
EMA recommends approval of trastuzumab biosimilar Herwenda
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) announced on 15 September 2023 that it had recommended granting of marketing authorization for the trastuzumab biosimilar Herwenda, 150 mg, for intravenous use.
CVS’ Cordavis to launch Sandoz’s Hyrimoz (adalimumab)
In August 2023, CVS Health Corp announced it has launched a new company called Cordavis that will work directly with manufacturers to commercialize and co-produce biosimilar medicines for the US market.
IBD barriers: an analysis of Latin America
A comprehensive review conducted by Queiroz et al. in 2023 [1] explores the barriers to accessing IBD care throughout Latin America, a large and diverse region used to describe South America, Central America, Mexico and the islands of the Caribbean. Each one of these regions/countries carries a different cultural and historical background, diverse political systems and a distinct healthcare system.
FDA approves first natalizumab biosimilar Tyruko for MS
On 24 August 2023, the US Food and Drug Administration approved Tyruko (natalizumab-sztn) as the first biosimilar to Tysabri (natalizumab) for the treatment of adults with relapsing forms of multiple sclerosis (MS). Tyruko is first-of-its-kind biosimilar natalizumab to receive approval in the US.