Recent court rulings have put the US’ ‘skinny label’ approval pathway at risk. Now, a research letter published in JAMA Internal Medicine [1] has found approval and marketing of skinny label biosimilars have led to billions of dollars in savings to Medicare.
Study investigates success of US ‘skinny label’ approval pathway
Biosimilars/Research
|
Posted 14/07/2023
 0
Post your comment
0
Post your comment
Biosimilar market entry is often delayed due to steps taken by originator drug manufacturers that can draw out patents for many years. In the US, skinny labelling was introduced to help generics and biosimilars manufacturers avoid originator manufacturer patent ‘walls’, or ‘thickets’. These walls can be created every time a drug is approved for a new indication (such as a new disease) or a new use (such as in paediatric patients). This can add another 20 years to a patent lifetime and block biosimilar market entry.
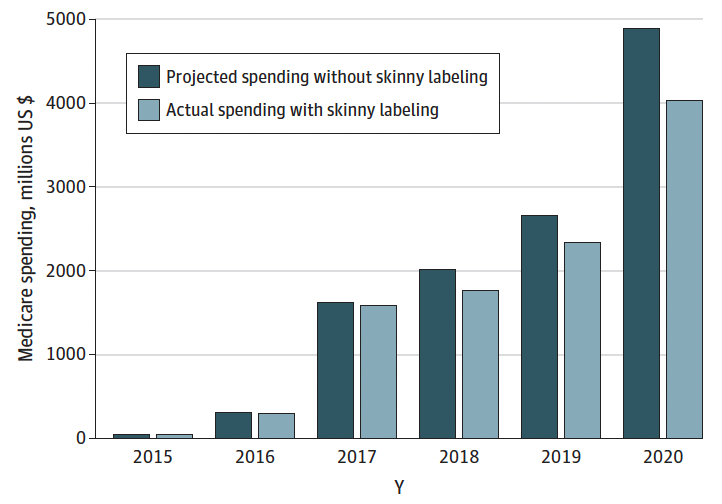
Skinny labelling, as approved by US congress, allows generics manufacturers to market a product following approval for the uses of the drug that are out of patent exclusivity. As such, the biosimilars company does not have to wait for all existing patents to expire and this also negates the need for them to attempt to invalidate patents in court [2]. The JAMA letter found that use of skinny labelling led to competition between five biosimilars and their skinny label biosimilars that saved Medicare an estimated US$1.5 billion during 2015‒2020, see Figure 1. It also highlights that, without threat, savings could continue to increase with skinny label biosimilars of blockbuster drugs such as Humira becoming available in coming years.
Figure 1: Estimated Medicare savings from skinny-label biosimilars, 2015 to 2020
However, recent court rulings, such as that which saw Teva lose the battle against GSK over the skinny labelling of Coreg, put the possible Medicare savings at risk. Here, Teva was requested to pay over US$20 million in damages due to patent infringement [2]. The JAMA letter notes that skinny labels are an important strategy for biosimilar and generic drugs, increasing the affordability and accessibility of medications across the US. It warns that originator manufacturers will continue to pursue new and complex strategies to protect their patents and exclusivities. The US congress needs to step in and enact additional legislation that reaffirms and strengthens biosimilar and generic drug skinny labelling.
Related articles
Lawsuits and US$450 million payout for price fixing and delayed generics entry
FDA voices concerns around drug patents and competition
European Court of Justice backs UK in pay-for-delay fine against GSK
|
LATIN AMERICAN FORUM View the latest headline article: Actualización de la declaración de posición de GADECCU sobre los biosimilares Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: Actualización de la declaración de posición de GADECCU sobre los biosimilares !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. Alexander C, Egilman BA, Victor L. Frequency of approval and marketing of biosimilars with a skinny label and associated Medicare savings. JAMA Internal Med. 2023;183(1):82-4.
2. GaBI Online - Generics and Biosimilars Initiative. Generic ‘skinny’ labelling under threat in the US [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2023 Jul 14]. Available from: www.gabionline.net/policies-legislation/generic-skinny-labelling-under-threat-in-the-us
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment