Biosimilars
Switches between biosimilars and their reference products
Biologicals are the fastest-growing class of medications in the United States and account for a substantial and growing portion of healthcare costs. The Biologics Price Competition Act of 2009 created an abbreviated approval pathway for the US Food and Drug Administration (FDA) to help provide patients with greater access to safe and effective biological products. As of 1 November 2023, FDA has approved 44 biosimilar products, 7 of which are interchangeable biosimilars. These products can be used to treat many conditions such as chronic skin and bowel diseases, arthritis, kidney conditions, diabetes, multiple sclerosis, macular degeneration, and cancer.
EC approval of natalizumab, aflibercept and tocilizumab biosimilars
In September 2023, the European Commission (EC) granted marketing authorization for the biosimilars of Tyruko (natalizumab), Yesafili (aflibercept), and Tyenne (tocilizumab).
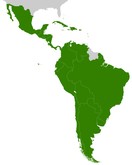
Latin America's biosimilars market: regulatory, institutional, and technological aspects
According to a study led by Bas (2023), the Latin American (LA) biosimilars market is positioned for substantial growth over the next decades as a cost-effective alternative to expensive patented biomolecules [1].
EMA recommends approval of first ustekinumab biosimilar Uzpruvo
On 9 November 2023, the European Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a marketing authorisation for the medicinal product Uzpruvo (ustekinumab), a biosimilar of reference product Stelara, intended for the treatment plaque psoriasis, including paediatric plaque psoriasis, psoriatic arthritis and Crohn’s disease in adults.
FDA approves first interchangeable ustekinumab biosimilar Wezlana
On 31 October 2023, the US Food and Drug Administration (FDA) approved Wezlana (ustekinumab-auub) as a biosimilar to and interchangeable with Stelara (ustekinumab) for multiple inflammatory diseases.
Canada’s Prince Edward Island adopts biosimilars switching policy
Canada’s Prince Edward Island (PEI) becomes the 11th Canadian jurisdiction to announce the adoption of biosimilars switching policies.
Impact of trastuzumab biosimilars use in metastatic HER2-positive breast cancer
A review conducted by Sarder and Ahmad attempted to describe and analyse the studies published in peer-reviewed literature on the use of trastuzumab biosimilars for patients with HER2-positive breast cancer, including their efficacy, safety, pharmacokinetic and pharmacodynamic properties [1].
Alvotech biosimilars: FDA ustekinumab application rejection; adalimumab interchangeability designation re-submission
In October 2023, Iceland-based biosimilar manufacturer Alvotech, announced that the US Food and Drug Administration (FDA) has issued a complete response letter (CRL) for Alvotech’s Biologics License Application (BLA) for AVT04, a biosimilar candidate to Stelara (ustekinumab). As such, the application was not approved. This follows Alvotech’s September 2023 resubmission of a BLA for their high-concentration biosimilar formulation to AbbVie’s blockbuster arthritis treatment Humira (adalimumab), AVT02 [1], following receipt of a similar CRL.
Japanese approval for first ustekinumab biosimilar
Iceland-based biosimilar manufacturer Alvotech announced on 25 September 2023 that its commercialization partner in Japan, Fuji Pharma Co Ltd (Fuji) had received marketing approval from Japan’s medicines regulatory agency, the Pharmaceuticals and Medical Devices Agency (PMDA), for its ustekinumab biosimilar AVT04 in Japan.
ASCO policy statement on biosimilars and interchangeables in oncology updated
The American Society of Clinical Oncology (ASCO) has released a new policy statement on the use of biosimilar and interchangeable products in oncology.
The statement proposes guidance and recommendations for stakeholders, including manufacturers, payers, regulatory agencies, clinicians, and patients, to ensure equitable access to high-quality care and to address challenges to biosimilar uptake [1].