Transplant coordinators associate the availability of multiple generic immunosuppression therapies with increased patient confusion, according to a study by Parker and co-authors [1].
Immunosuppression regimens containing mycophenolate mofetil (MMF) and tacrolimus (TAC) are used to prevent acute rejection in solid organ transplant recipients. The first generic MMF and TAC were approved in July 2008 and August 2009, respectively, and there are now 10 generics manufacturers of MMF and four generics manufacturers of TAC.
This observational, cross-sectional, multicentre study was carried out via an online survey of transplant coordinators in the US from August 2010 to September 2010, to find out transplant coordinators’ perception of workload over time.
Forty-six of the 106 transplant centres contacted (43%) completed or partially completed the online survey. Usable information was obtained from 28 transplant centres, which corresponded to 34 transplant coordinators.
Fifteen per cent of transplant coordinators reported that more than half of transplant recipients had switched from brand-name to generic TAC and 38% reported that more than half of recipients switched from brand-name to generic MMF.
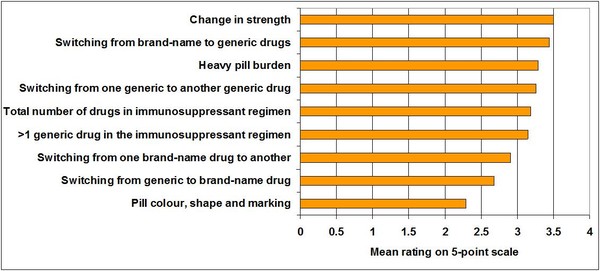
The results indicated that three out of every four patients (76.5%) are often confused or sometimes confused about their immunosuppression therapy. The top four reasons for confusion were change in strength, switching from brand-name to generic medications, heavy pill burden and switching from one generic to another generic drug. These were closely followed by the total number of medications in immunosuppressant regimen, having more than one generic medication in the immunosuppressant regimen and switching from one brand-name to another brand-name treatment, see Figure 1.
Figure 1: Factors contributing to patient confusion during immunosuppressant therapy
Perhaps surprisingly, the colour, shape and marking of the pills are given the lowest rating as being likely to cause confusion in patients on immunosuppressant therapy. This is in contrast to what patients have reported previously, and in fact these physical characteristics have also been shown to influence compliance [2]. Differences in the shape and colour of generics may reduce compliance when brand-name and generic drugs are switched frequently, according Maliepaard et al. [3]; and there have been demands for generics to have the same or at least similar shape and colour as the brand-name medication [4]. Although other research has shown that price, familiarity, and availability were more important for patients in their choice of prescription medicines [5].
The authors concluded that increases in patient confusion could cause potential interruptions in therapy, introducing additional risks to a population that already takes multiple medications.
However, perhaps the issue is not generics, but generics awareness and the continued need for patients to be made more aware of the safety and efficacy of generics. A previous study has shown that patients with poor awareness of generic medicines, when offered a substitute, were more likely to become confused and suspicious [6].
Conflict of interest
The study was funded by Novartis Pharmaceuticals Corporation.
Editor’s comment
GaBI Journal is publishing manuscripts within the educational series on Generic Immunosuppressants in Transplantation, with Professor Teun van Gelder as the Guest Editor.
If you are interested in contributing a research paper on equivalence of generic immunosuppressants to GaBI Journal, please send us your submission here.
Related article
FDA to release guidance on generic tablet size
References
1. Parker K, Zagadailov EA, Bruno AS, Wiland AM. Transplant coordinators' perceived impact of availability of multiple generic immunosuppression therapies on patients, workload, and posttransplant maintenance therapy. J Transplant. 2013;2013:897434.
2. GaBI Online - Generics and Biosimilars Initiative. Compliance with generic drug use among Pakistani immigrants [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Oct 4]. Available from: www.gabionline.net/Generics/Research/Compliance-with-generic-drug-use-among-Pakistani-immigrants
3. Maliepaard M, Leufkens H, Yu Y. Equivalence of generic medicines in general and immunosuppressants in particular – a regulatory opinion on switching of cyclosporin, tacrolimus and mycophenolate mofetil. Generics and Biosimilars Initiative Journal (GaBI Journal) 2013;2(2):86-90. doi:10.5639/gabij.2013.0202.019
4. van Gelder T. Why bioequivalence and unconditional interchangeability of generic drugs are not the same. Generics and Biosimilars Initiative Journal (GaBI Journal) 2013;2(2):83-5. doi:10.5639/gabij.2013.0202.020
5. GaBI Online - Generics and Biosimilars Initiative. Factors important for generics substitution in Finland [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Oct 4]. Available from: www.gabionline.net/Generics/Research/Factors-important-for-generics-substitution-in-Finland
6. GaBI Online - Generics and Biosimilars Initiative. Generic substitution causing confusion [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2013 Oct 4]. Available from: www.gabionline.net/Generics/Research/Generic-substitution-causing-confusion
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2013 Pro Pharma Communications International. All Rights Reserved.








 0
0









Post your comment