Criteria for PD biomarkers intended to support a demonstration of biosimilarity are inherently different from criteria for surrogate biomarkers used to support new drug approvals.
Key considerations for PD biomarkers in evaluating biosimilarity
Home/Reports
|
Posted 17/12/2021
 0
Post your comment
0
Post your comment

Dr Peter Stein, Director of the Office of New Drugs at the Center for Drug Evaluation and Research (CDER) of the US Food and Drug Administration (FDA), gave a presentation entitled ‘An update on the FDA biosimilar program: progress and directions, 2021’ during the DIA Biosimilars Conference 2021. In his presentation Dr Stein outlined the key considerations for PD biomarkers in the evaluation of biosimilarity [1].
According to Dr Stein, the key points when considering PD biomarkers in the evaluation of biosimilarity are that:
• PD biomarkers for biosimilars do not need to be surrogate endpoints for clinical efficacy outcomes
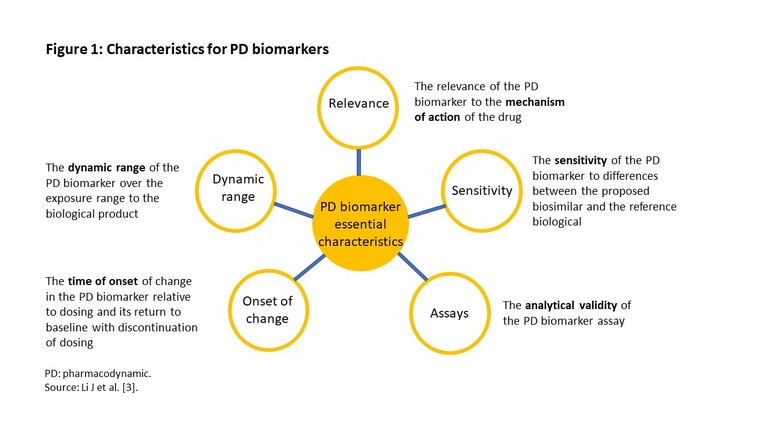
• Characterization of PD biomarkers according to the 5 key characteristics is critical to assess their suitability
• A single clinical pharmacology study can assess both PK and PD similarity if designed appropriately
• Utilization of PD biomarkers can eliminate the need for comparative clinical efficacy studies, streamlining biosimilar development
In its guidance, FDA has described five characteristics for PD biomarkers to assist sponsors planning to use PD biomarkers as a component of a biosimilar development programme [2], see Figure 1.
Conflict of interest
The author of the presentation [1] did not provide any conflict-of-interest statement.
Related articles
The ‘positioning’ of PD biomarkers in evaluating biosimilarity
The role of PD biomarkers in biosimilarity
PD biomarkers for biosimilar development and approval
| LATIN AMERICAN FORUM The new section of the ‘Latin American Forum’ on GaBI has been launched. The objective of this new section is to provide you with all the latest news and updates on developments of generic and biosimilar medicines in Latin America in Spanish. View this week’s headline article: Guía evaluación de comparabilidad de medicamentos biológicos en Colombia Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative. LATIN AMERICAN FORUM Se ha lanzado la nueva sección del ‘Foro Latinoamericano’ sobre GaBI. El objetivo de esta nueva sección es brindarle las últimas noticias y actualizaciones sobre desarrollos de medicamentos genéricos y biosimilares en América Latina en español. Vea el artículo principal de esta semana: Guía evaluación de comparabilidad de medicamentos biológicos en Colombia Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
References
1. An update on the FDA biosimilar program: progress and directions, 2021. DIA Biosimilars, Virtual Conference, 5-6 October 2021.
2. GaBI Online - Generics and Biosimilars Initiative. US guidelines for biosimilars [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2021 Dec 17]. Available from: www.gabionline.net/guidelines/US-guidelines-for-biosimilars
3. Li J, Florian J, Campbell E, et al. Advancing biosimilar development using pharmacodynamic biomarkers in clinical pharmacology studies. Clin Pharmacol Ther. 2020;107(1):40-2.
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2021 Pro Pharma Communications International. All Rights Reserved.
Source: Duke, US FDA
Guidelines
US guidance to remove biosimilar comparative efficacy studies
New guidance for biologicals in Pakistan and Hong Kong’s independent drug regulatory authority
Policies & Legislation
EU accepts results from FDA GMP inspections for sites outside the US
WHO to remove animal tests and establish 17 reference standards for biologicals
EU steps closer to the ‘tailored approach’ for biosimilars development

Home/Reports Posted 21/11/2025
Advancing biologicals regulation in Argentina: from registration to global harmonization

Home/Reports Posted 10/10/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment