On 27 March 2015, the European Medicines Agency (EMA) published its reflection paper for follow-on versions of iron-based nano-colloidal products. The agency first released the draft reflection paper for follow-on versions of iron-based nano-colloidal products for a three-month consultation period in September 2013.
EMA issues reflection paper for follow-on versions of iron-based nano-colloidal products
Non‐Biological Complex Drugs/Guidelines
|
Posted 05/11/2015
 0
Post your comment
0
Post your comment

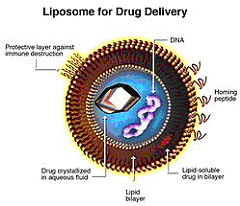
EMA tends to refer to biosimilars regulations when considering ‘follow-on’ versions of non-biological complex drugs (NBCDs), such as iron carbohydrate complexes, liposomal drugs and glatiramoids.
Like biologicals, NBCDs consist of different (closely related) structures that cannot be fully quantitated, characterized or described by (physico-)chemical analytical tools. The composition and quality of NBCDs are dependent on the manufacturing process and controls – just as is the case with biologicals. Therefore, approval of follow-on NBCDs via generics pathways may not be appropriate and use of biosimilars pathways for non-biologicals is also not appropriate.
Via this reflection paper, which is available on the EMA website, EMA sets out additional data requirements for follow-on versions of iron-based nano-colloidal products used to treat iron deficiency. These extra requirements have been based on the fact that quality characterization on its own would not provide sufficient assurance of the similarity between the follow-on version and the originator product, even if the quality tests performed show similarity. In the context of such iron-based preparations, EMA has decided that a ‘weight of evidence approach’ including data from quality, non-clinical and human pharmacokinetic studies is required.
Reflection paper on follow-on versions of iron-based nano-colloidal products
Date: 26 March 2015
http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184922.pdf
EMA has also adopted a reflection paper for intravenous liposomal products [1].
Related articles
EU guidelines for biosimilars
Reference
1. GaBI Online - Generics and Biosimilars Initiative. EU guidelines for follow-on NBCDs [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Nov 6]. Available from:
www.gabionline.net/Non-Biological-Complex-Drugs/Reports/Regulations-for-follow-on-NBCDs
Source: EMA
FDA issues final guidance on liposome drug products
Non‐Biological Complex Drugs/Guidelines Posted 27/04/2018
FDA includes follow-on versions in its new cyclosporine guideline

Non‐Biological Complex Drugs/Guidelines Posted 29/04/2016
FDA includes follow-on versions in its new liposome guideline

Non‐Biological Complex Drugs/Guidelines Posted 26/02/2016
The best selling biotechnology drugs of 2008: the next biosimilars targets






Post your comment