Non‐Biological Complex Drugs
FDA issues final guidance on liposome drug products
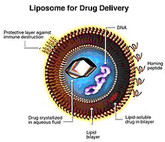
On 4 April 2018, the US Food and Drug Administration (FDA) issued a new draft guidance document for non-biological complex drugs (NBCDs). The finalized guidance covers what applicants should submit for new drug applications (NDAs) and abbreviated new drug applications (ANDAs) for liposome products.
FDA approves 40 mg follow-on version of glatiramer acetate
Sandoz, the generics division of Novartis, announced on 13 February 2018 the approval of its 40 mg follow-on version of glatiramer acetate by the US Food and Drug Administration (FDA).
Mylan launches first follow-on glatiramer acetate in the UK
US generics giant Mylan announced on 23 January 2018 that it had launched Brabio, the first follow-on version of Teva Pharmaceutical’s (Teva) Copaxone (glatiramer acetate) in the UK.
Biosimilars and follow-on NBCDs for MS in Europe, the US and Canada
The advent of biological medicines has significantly transformed the landscapes of many disease spaces and improved the lives of millions around the world. However, the structural complexity and sensitivity of such products result in a high price tag, adding to already financially strained healthcare systems. As these and other expensive complex drugs lose market exclusivity, stakeholders eagerly await the arrival of lower cost alternatives, such as biosimilars and follow-on non-biological complex drugs (NBCDs). Nevertheless, stakeholders remain uncertain about key issues which have resulted in heterogeneous reimbursement policies and varying levels of biosimilar uptake and differences in the approval processes for follow-on NBCDs between different markets.
Impax announces FDA approval of follow-on sevelamer carbonate
US generics maker Impax Laboratories (Impax) announced on 23 October 2017 that it had received final US Food and Drug Administration (FDA) approval for its abbreviated new drug application (ANDA) for a follow-on version of Renvela (sevelamer carbonate).
FDA approves follow-on version of sevelamer
Indian generics maker Aurobindo Pharma (Aurobindo) announced on 15 June 2017 that it had received US Food and Drug Administration (FDA) approval for its follow-on version of sevelamer.
Challenges in the regulation of NBCDs
Author Leonie Hussaarts and colleagues discuss in a white paper the regulatory frameworks in use worldwide to approve non-biological complex drugs (NBCDs) and their follow-on versions [1].
Scientific and regulatory considerations for follow-on versions of complex drug products containing nanomaterials
In this article [1], US Food and Drug Administration (FDA) scientists give a comprehensive overview of the nanomaterials presently available in the US and the challenges encountered when considering approval of generic (or follow-on) versions of these often complex drug products. More specifically, these challenges encompass ‘establishing active ingredient sameness, equivalence in the drug product physicochemical properties and equivalence in thein vivodrug exposure profiles between innovator and generic drug products’.
Equivalence of complex drug products
Complex drug products and their generic (or follow-on) versions was the subject discussed at the New York Academy of Sciences symposium on Equivalence of Complex Drug Products: Scientific and Regulatory Challenges [1].
Follow-up studies needed to ensure safety for follow-on NBCDs
Product-specific testing to determine therapeutic equivalence has been an approach used by the US Food and Drug Administration (FDA) since 1984, including products with unique features. In a recent paper Kesselheim and Gagne [1] discussed a number of aspects related to NBCDs.