Non‐Biological Complex Drugs/
Guidelines
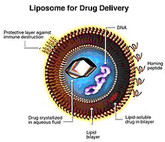
FDA issues final guidance on liposome drug products
On 4 April 2018, the US Food and Drug Administration (FDA) issued a new draft guidance document for non-biological complex drugs (NBCDs). The finalized guidance covers what applicants should submit for new drug applications (NDAs) and abbreviated new drug applications (ANDAs) for liposome products.
US guidelines for follow-on NBCDs
Last update: 1 June 2018
The regulatory body for approval of medicines in the US is the Food and Drug Administration (FDA).
FDA includes follow-on versions in its new cyclosporine guideline
In February 2016, the US Food and Drug Administration (FDA) issued a new draft guidance document for non-biological complex drugs (NBCDs). The new draft guidance on cyclosporine drug products replaces draft guidance published in March 2015.
FDA includes follow-on versions in its new liposome guideline
On 29 October 2015, the US Food and Drug Administration (FDA) issued a new draft guidance document for non-biological complex drugs (NBCDs). The new draft guidance on liposome drug products replaces draft guidance published in August 2002.
EU guidelines for follow-on NBCDs
Last update: 22 January 2016
The regulatory body for approval of medicines in the European Union (EU) is the European Medicines Agency (EMA). The agency is responsible for the scientific evaluation of medicines developed by pharmaceutical companies for use in the EU.
EMA issues reflection paper for follow-on versions of iron-based nano-colloidal products
On 27 March 2015, the European Medicines Agency (EMA) published its reflection paper for follow-on versions of iron-based nano-colloidal products. The agency first released the draft reflection paper for follow-on versions of iron-based nano-colloidal products for a three-month consultation period in September 2013.