Non‐Biological Complex Drugs
Glatiramoid follow-on NBCDs
Follow-on versions of glatiramoid non-biological complex drugs (NBCDs) was a subject discussed at the International Symposium on the Scientific and Regulatory Advances in Complex Drugs, which took place in Budapest, Hungary on 27–28 October 2014 [1].
FDA includes follow-on versions in its new liposome guideline
On 29 October 2015, the US Food and Drug Administration (FDA) issued a new draft guidance document for non-biological complex drugs (NBCDs). The new draft guidance on liposome drug products replaces draft guidance published in August 2002.
EU guidelines for follow-on NBCDs
Last update: 22 January 2016
The regulatory body for approval of medicines in the European Union (EU) is the European Medicines Agency (EMA). The agency is responsible for the scientific evaluation of medicines developed by pharmaceutical companies for use in the EU.
GDUFA regulatory priorities for 2016 include complex drugs
As part of the Generic Drug User Fee Amendments (GDUFA) of 2012, the US Food and Drug Administration (FDA) committed to prepare a yearly list of regulatory science priorities for generics based on input from industry and other stakeholders.
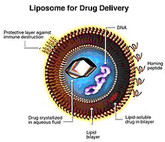
Liposomal follow-on NBCDs
Follow-on versions of liposomal non-biological complex drugs (NBCDs) was a subject discussed at the International Symposium on the Scientific and Regulatory Advances in Complex Drugs, which took place in Budapest, Hungary on 27–28 October 2014 [1].
Regulations for follow-on NBCDs
Regulations for follow-on versions of non-biological complex drugs (NBCDs) in Europe and the US were discussed at the International Symposium on the Scientific and Regulatory Advances in Complex Drugs, which took place in Budapest, Hungary on 27–28 October 2014 [1].
Status and regulatory issues surrounding follow-on NBCDs
Status and outstanding regulatory issues for follow-on versions of non-biological complex drugs (NBCDs) were discussed at the International Symposium on the Scientific and Regulatory Advances in Complex Drugs, which took place in Budapest, Hungary on 27–28 October 2014 [1].
Follow-on glatiramer acetate (M356) claimed as equivalent to Copaxone
A multi-dimensional evaluation of the equivalence of a follow-on glatiramer acetate (M356) and the brand-name originator version (Copaxone) has been reported to show equivalence of biological and physicochemical properties, with no significant differences in the structure and function of the two products [1].
EMA issues reflection paper for follow-on versions of iron-based nano-colloidal products
On 27 March 2015, the European Medicines Agency (EMA) published its reflection paper for follow-on versions of iron-based nano-colloidal products. The agency first released the draft reflection paper for follow-on versions of iron-based nano-colloidal products for a three-month consultation period in September 2013.
FDA approves first follow-on version of glatiramer acetate
The US Food and Drug Administration (FDA) announced on 16 April 2015 the approval of the first follow-on version of glatiramer acetate injection used to treat patients with relapsing forms of multiple sclerosis (MS).