The adoption of biosimilars is lower in regions experiencing low social and governmental trust, finds a recent study that focused on uptake in provinces of Italy and Germany [1].
Low biosimilar uptake in regions of low social and political trust
Biosimilars/Research
|
Posted 23/03/2023
 0
Post your comment
0
Post your comment

Despite the implementation economic incentives across Europe, the study reports that the adoption of biosimilars is below expectations. These biological products, if adopted, can offer a route to more affordable treatment of various conditions. The authors, who are based at the University of California, Berkeley in the US, set out to determine the role of behavioural factors such as citizens’ trust in one another (social trust) and trust in government policymakers (political trust), when it comes to biosimilar adoption.
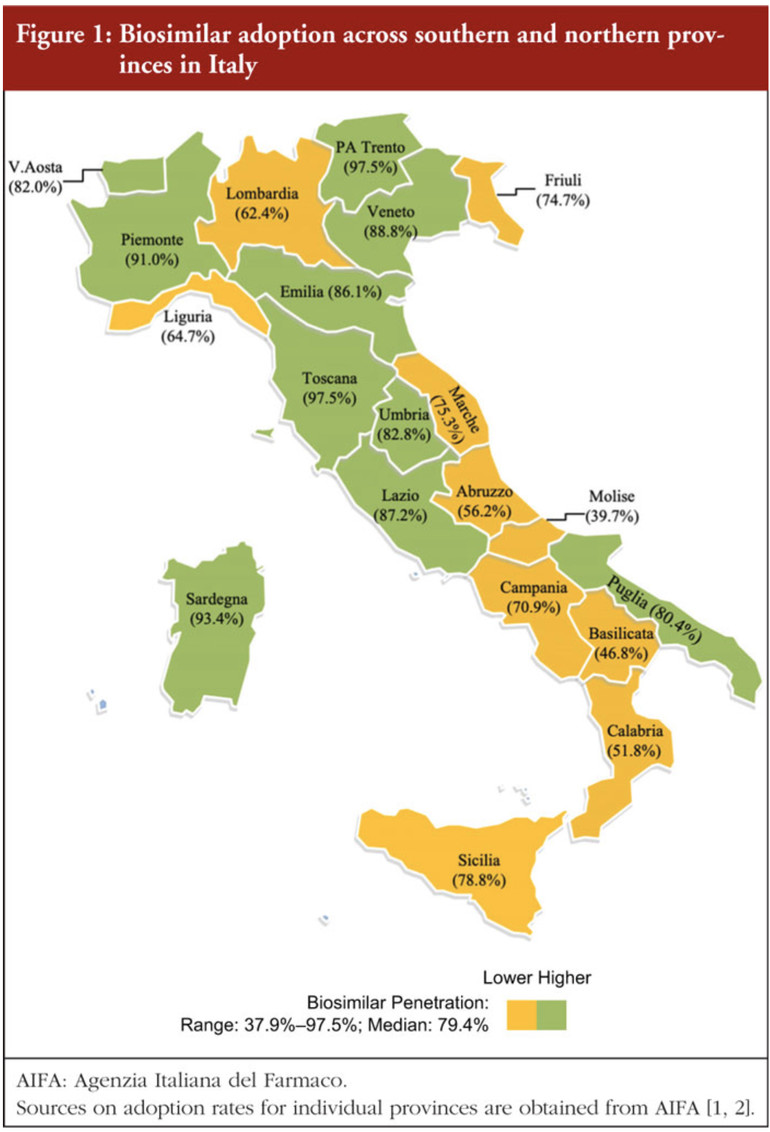
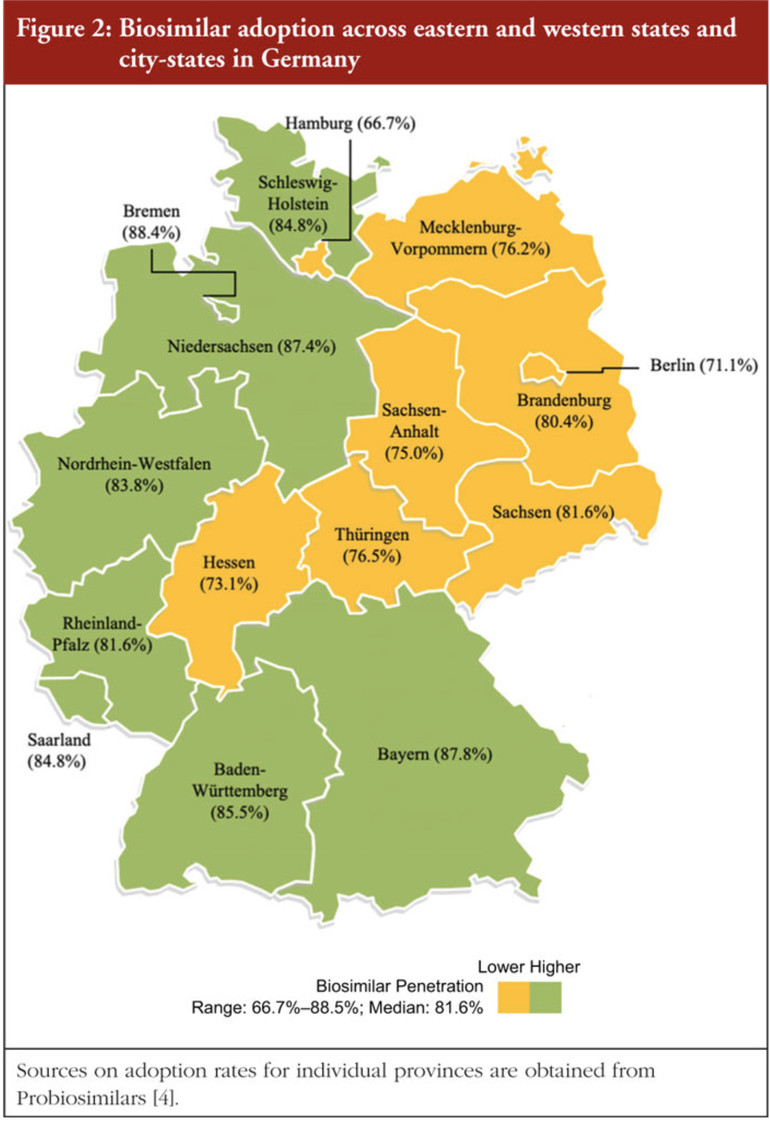
The study by Professor James C Robinson presented data on biosimilar adoption from provinces in Italy and Germany in 2020 (Figures 1 and 2). The authors used historical metrics based on various factors to code for social trust in Italy and Germany. Political trust was coded using the Quality of Government Index (QGI).
The study included data on two biologicals used to treat chronic immunological conditions and three biologicals for acute cancer treatment, and their 20 biosimilars. Multivariable methods were used to ascertain the association between adoption, social trust, political trust and income per capita.
Overall, it was seen that the adoption of biosimilars was much lower in regions suffering from low social trust and low trust in government. The author emphasized that penetration fell below the national median in seven out of eight provinces in southern Italy and in all seven provinces in eastern Germany. Robinson stated that rates of adoption are 21.5 percentage points higher in northern than in southern Italy and 5.2 points higher in western than in eastern Germany.
The author concludes that the study highlights the important role of social trust and trust in government when it comes to having trust in experts and institutions. In regions of Italy and Germany with historically disadvantaged populations, there are lower levels of trust and consequently lower levels of biosimilar uptake, this is thought to be because they are less inclined to believe they will benefit from budgetary advantages. It is suggested that incentives for biosimilar uptake need to be redesigned in such regions in a way that might enhance social and political trust.
Related articles
US vs Germany and Switzerland: US biosimilars market lags with higher prices
Challenges of biosimilar medicines in France
Medicines pricing conditions in Italy and Brazil: comparison of regulations
Successful increase of biosimilar adoption in a large integrated health delivery network
|
LATIN AMERICAN FORUM View the latest headline article: La adopción exitosa de biosimilares en Europa y EE. UU. Browse the news in the Latin American Forum! Register to receive the GaBI Latin American Forum newsletter. Inform colleagues and friends of this new initiative.
FORO LATINOAMERICANO Ver el último artículo de cabecera: La adopción exitosa de biosimilares en Europa y EE. UU. !Explore las noticias en el Foro Latinoamericano! Regístrese para recibir el boletín informativo GaBI Foro Latinoamericano. Informe a colegas y amigos sobre esta nueva iniciativa. |
Reference
1. Robinson JC. Social trust and regional variation in the adoption of biosimilars in Italy and Germany. Generics and Biosimilars Initiative Journal (GaBI Journal). 2022;11(3):87-8. doi:10.5639/gabij.2022.1103.015
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2023 Pro Pharma Communications International. All Rights Reserved.
News
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
What is the future for the US biosimilar interchangeability designation

Biosimilars/Research Posted 05/06/2025
Biosimilar clinical efficacy studies: are they still necessary?

Biosimilars/Research Posted 27/05/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets








Post your comment