India-based Ranbaxy Laboratories (Ranbaxy) announced on 1 December 2014 that it had launched its infliximab ‘similar biologic’, BOW015 in India. This is the first infliximab similar biologic to be launched in India.
First infliximab ‘similar biologic’ launched in India
Biosimilars/News
|
Posted 09/01/2015
 0
Post your comment
0
Post your comment
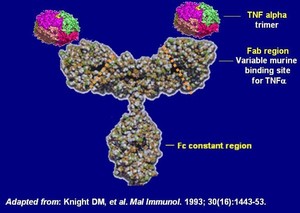
BOW015 is a similar biologic of Johnson & Johnson’s blockbuster drug Remicade, which was approved by the European Medicines Agency (EMA) in August 1999. Remicade is indicated for the treatment of inflammatory diseases including rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, ulcerative colitis, psoriatic arthritis and psoriasis.
BOW015 is being manufactured by Reliance Life Sciences at a facility in Mumbai. The drug has been launched under the brand name Infimab and is being introduced to the Indian market through a licensing partnership with US-based biologicals company Epirus Biopharmaceuticals (Epirus).
Rheumatoid arthritis is one of the most common chronic inflammatory diseases causing disability and is estimated to have a prevalence of 0.5−0.75% in India. Infimab will be available in India at a significant discount compared to the originator drug. In order to increase patient awareness and compliance Ranbaxy has launched a support programme: RAISE (Rheumatoid Ailment Infimab Support Endeavor).
According to Mr Rajeev Sibal, Vice President and Country Head-India Region, Ranbaxy, ‘Infimab offers a new opportunity in the management of conditions like rheumatoid arthritis. The product has been developed as per global standards and delivers a similar clinical outcome to the innovator.’ Results from phase I and phase III clinical trial results have shown the ‘bioequivalence’ and ‘clinical comparability’ of BOW015 to Remicade [1-3].
The world’s first monoclonal antibody biosimilar Remsima/Inflectra (infliximab) was approved in Europe in September 2013 [4]. Since then South Korean biotechnology company Celltrion has also gained approval for Remsima in 50 countries worldwide, including South Korea [5], Colombia [6], Canada and Japan [7]. The company has also applied for approval of Remsima in the US via the biosimilars pathway [8].
Editor’s comment
It should be noted that ‘similar biologics’ approved in India might not have been authorized following as strict a regulatory process as is required for approval of biosimilars in the European Union. The EMA (European Medicines Agency) regulatory requirements ensure the same high standards of quality, safety and efficacy for biosimilars as for originator biologicals, and also include a rigorous comparability exercise with the reference product.
Related articles
Phase I studies of infliximab and rituximab biosimilars demonstrate pharmacokinetic similarity
Biosimilar infliximab comparable to Remicade
‘Similar biologics’ approved and marketed in India
References
1. GaBI Online - Generics and Biosimilars Initiative. Positive phase I data for infliximab biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/Research/Positive-phase-I-data-for-infliximab-biosimilar
2. GaBI Online - Generics and Biosimilars Initiative. Positive phase III data for Epirus infliximab biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/Research/Positive-phase-III-data-for-Epirus-infliximab-biosimilar
3. GaBI Online - Generics and Biosimilars Initiative. Biosimilar infliximab comparable to Remicade [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/Research/Biosimilar-infliximab-comparable-to-Remicade
4. GaBI Online - Generics and Biosimilars Initiative. EC approves first monoclonal antibody biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/News/EC-approves-first-monoclonal-antibody-biosimilar
5. GaBI Online - Generics and Biosimilars Initiative. Biosimilar monoclonal antibody approved in Korea [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-monoclonal-antibody-approved-in-Korea
6. GaBI Online - Generics and Biosimilars Initiative. Remsima approved in Colombia [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/News/Remsima-approved-in-Colombia
7. GaBI Online - Generics and Biosimilars Initiative. Biosimilar infliximab receives approval in Japan and Turkey [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/News/Biosimilar-infliximab-receives-approval-in-Japan-and-Turkey
8. GaBI Online - Generics and Biosimilars Initiative. FDA receives application for monoclonal antibody biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2015 Jan 9]. Available from: www.gabionline.net/Biosimilars/News/FDA-receives-application-for-monoclonal-antibody-biosimilar
Permission granted to reproduce for personal and non-commercial use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Copyright – Unless otherwise stated all contents of this website are © 2015 Pro PharmaCommunications International. All Rights Reserved.
Source: Ranbaxy
Research
Reaching ESG goals in pharmaceutical development
What is the future for the US biosimilar interchangeability designation
General
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus
FDA approves aflibercept biosimilar Eydenzelt and label expansion for adalimumab biosimilar Yuflyma
FDA approves Poherdy (first interchangeable pertuzumab) and Armlupeg (pegfilgrastim) biosimilars

Biosimilars/News Posted 27/01/2026
EMA recommends approval for insulin glargine biosimilar Ondibta and denosumab biosimilar Osqay

Biosimilars/News Posted 16/01/2026
FDA approves denosumab biosimilars Osvyrti and Jubereq, Boncresa and Oziltus

Biosimilars/News Posted 07/01/2026
FDA approves aflibercept biosimilar Eydenzelt and label expansion for adalimumab biosimilar Yuflyma

Biosimilars/News Posted 05/12/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets






Post your comment