Amgen has been a leading biotechnology company since 1980. Amgen pioneered the development of novel products based on advances in recombinant DNA and molecular biology and launched the biotechnology industry’s first blockbuster medicines.
Competing biosimilars to Amgen’s biologicals
Biosimilars/General
|
Posted 30/11/2012
 0
Post your comment
0
Post your comment

Amgen has grown into a huge multinational based on its biotechnology products and currently employs approximately 17,000 people worldwide, with worldwide sales in 2011 of US$15.3 billion.
Amgen’s products have been used to treat life-threatening illnesses such as cancer, kidney disease, rheumatoid arthritis, as well as other serious illnesses in millions of patients around the world. Biological drugs can cost patients and payers up to 22 times more than traditional ‘chemical’ medications – some as much as US$400,000 a year.
Thus, enter the biosimilars – similar biological medicines – which can provide substantial savings to both patients and payers, ending Amgen’s domination of the biologicals market, which has lasted since 1980 in the US.
Europe approved its first biosimilar back in 2006 and currently there are 13 biosimilars approved for use in Europe [1]. The US now has a legal pathway, since the approval of the Biologics Price Competition and Innovation Act, which was signed into law on 23 March 2010 by President Barack Obama [2]. Draft guidance has also now been issued by FDA [3], although there are no timelines as yet for its implementation.
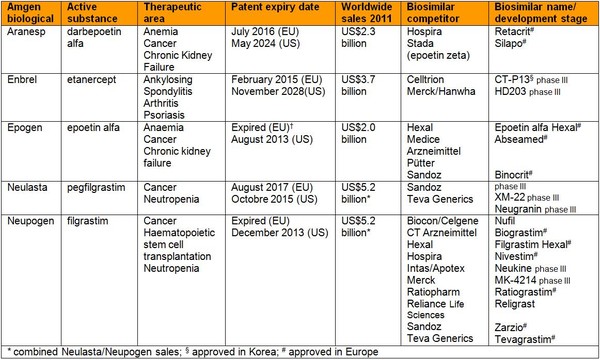
The biosimilar competitors expected to challenge Amgen’s biologicals reign are listed in Table 1, along with patent expiry dates and biological sales.
Table 1: Amgen’s biologicals and competing biosimilars
Amgen, as one of the world’s largest biologicals manufacturers, has also decided to pursue biosimilars. The biotech giant made a deal with generic drugmaker Watson in December 2011 for the development and commercialization of several cancer antibody biosimilars [4]. Then in July 2012, Amgen and Watson entered into a global licensing agreement with Netherlands-based biotech Synthon for the clinical development and testing of Synthon’s biosimilar version of Roche’s blockbuster breast cancer and gastric cancer drug Herceptin (trastuzumab) [5]. While in April 2012 another deal with Clinical Research Organization PRA was announced for a series of phase III studies to develop several biosimilar drugs [6].
Related articles
Amgen welcomes biosimilar monoclonal antibody guideline
New Amgen Enbrel patent could block biosimilars until 2028
References
1. GaBI Online - Generics and Biosimilars Initiative. Biosimilars approved in Europe [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Nov 30]. Available from: www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe
2. GaBI Online - Generics and Biosimilars Initiative. US healthcare reform [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Nov 30]. Available from: www.gabionline.net/Policies-Legislation/US-healthcare-reform
3. GaBI Online - Generics and Biosimilars Initiative. FDA finally issues draft biosimilar guidance [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Nov 30]. Available from: www.gabionline.net/Guidelines/FDA-finally-issues-draft-biosimilar-guidance
4. GaBI Online - Generics and Biosimilars Initiative. Amgen finally jumps on biosimilars bandwagon [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Nov 30]. Available from: www.gabionline.net/Biosimilars/News/Amgen-finally-jumps-on-biosimilars-bandwagon
5. GaBI Online - Generics and Biosimilars Initiative. Deal struck over development of Herceptin biosimilar [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Nov 30]. Available from: www.gabionline.net/Biosimilars/News/Deal-struck-over-development-of-Herceptin-biosimilar
6. GaBI Online - Generics and Biosimilars Initiative. Amgen enters biosimilars deal with PRA [www.gabionline.net]. Mol, Belgium: Pro Pharma Communications International; [cited 2012 Nov 30]. Available from: www.gabionline.net/Pharma-News/Amgen-enters-biosimilars-deal-with-PRA
Permission granted to reproduce for personal and educational use only. All other reproduction, copy or reprinting of all or part of any ‘Content’ found on this website is strictly prohibited without the prior consent of the publisher. Contact the publisher to obtain permission before redistributing.
Source: Amgen, Forbes, GaBI Online
Research
Reaching ESG goals in pharmaceutical development
What is the future for the US biosimilar interchangeability designation
News
EMA recommends approval for teriparatide biosimilar Zandoriah
FDA approves third interchangeable ranibizumab biosimilar Nufymco
Most viewed articles
The best selling biotechnology drugs of 2008: the next biosimilars targets
Global biosimilars guideline development – EGA’s perspective
Related content
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars
Chinese biosimilars go global: growth, partnerships, and challenges
Stelara biosimilars enter US market with 85% discount in 2025
IFPMA publishes position on pharmacy-mediated substitution for biosimilars
Samsung Bioepis wins Pyzchiva case; Regeneron patent rulings threaten foreign biosimilars

Biosimilars/General Posted 30/07/2025
Chinese biosimilars go global: growth, partnerships, and challenges

Biosimilars/General Posted 30/04/2025
IFPMA publishes position on pharmacy-mediated substitution for biosimilars

Biosimilars/General Posted 21/03/2025
The best selling biotechnology drugs of 2008: the next biosimilars targets







Post your comment