Biosimilars/General
Biosimilars in Australia – a-flagging and sustainability
Australia first introduced guidelines for biosimilars back in August 2008 when it adopted a number of guidelines from the EU on similar biological medicinal products [1].
Biosimilars of rituximab
Last update: 8 January 2021
Rituximab is a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of immune system B cells. Rituximab destroys B cells and is therefore used to treat diseases that are characterized by excessive number of B cells, overactive B cells or dysfunctional B cells. This includes many lymphomas, leukaemias, transplant rejection and autoimmune disorders.
Biosimilar advances for Samsung Bioepis
Korea-based Samsung Bioepis (Samsung and Biogen’s joint venture) has announced several advances related to its infliximab, denosumab and ranibizumab biosimilars. These announcements come after the company revealed soaring sales figure in Europe.
Biosimilars of denosumab
Last update: 11 December 2020
Denosumab is a humanized monoclonal antibody that is an inhibitor of the receptor activator of nuclear factor kappa-B ligand (RANKL), which works by preventing the development of osteoclasts which are cells that break down bone. It is used for the treatment of osteoporosis, treatment-induced bone loss, metastases to bone and giant cell tumour of bone.
Biosimilars of teriparatide
Last update: 11 December 2020
Teriparatide is a recombinant form of parathyroid hormone (PTH). Teriparatide is identical to a portion of human PTH and intermittent use activates osteoblasts more than osteoclasts, which leads to an overall increase in bone. This makes it an effective anabolic, i.e., bone growing, agent. It is therefore used for the treatment of osteoporosis in postmenopausal women and men at high risk for fracture and for glucocorticoid-induced osteoporosis in men and postmenopausal women.
Genentech sues Centus over Avastin biosimilar
Genentech Inc. filed a complaint against Centus in the United States (US) state of Texas on 15 November 2020. It is alleged that Centus’s Equidacent, a biosimilar to Genentech’s Avastin (bevacizumab), infringes 10 US patents.
Biosimilars of ranibizumab
Last update: 4 December 2020
Ranibizumab is a monoclonal antibody fragment created from the same parent mouse antibody as bevacizumab. Ranibizumab inhibits angiogenesis (the formation of new blood vessels) by inhibiting vascular endothelial growth factor A (VEGF-A), a mechanism similar to bevacizumab [1].
Biosimilars of tocilizumab
Last update: 4 December 2020
Tocilizumab is a humanized monoclonal antibody against the interleukin-6 receptor (IL-6R). It is an immunosuppressive drug used mainly for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children. In Japan, tocilizumab is also approved for the treatment of Castleman’s disease, a rare benign tumour of B cells.
Biosimilar toolkit for cancer patients
The European Cancer Patient Coalition (ECPC) has launched a biosimilars e-module, or toolkit [1]. This aims to teach patients about the value of biosimilars.
Biosimilars of infliximab
Last update: 27 November 2020
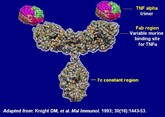
Infliximab is a chimeric monoclonal antibody against tumour necrosis factor alpha (TNF-α). It is used to treat autoimmune diseases, such as ankylosing spondylitis, Crohn’s disease, psoriasis, psoriatic arthritis, rheumatoid arthritis, and ulcerative colitis.